EDITORIALS
So first to Extended-Release Guanfacine for Hyperactivity in Children With Autism Spectrum Disorder – by Lawrence Scahill, M.S.N., Ph.D., et al. I spent four or five years in my retirement volunteering at a local Child and Adolescent agency. I saw a number of special needs kids, including the Autistic Spectrum variety. Many of them do have the Attention/Hyperactivity diathesis described here, and the question of medication was often in the wind. I had seen several kids on Tenex where it helped a lot and several where the side effect burden was prohibitive, so when I saw this article, I was drawn to it by interest. I wish they had done the study as proposed with a stimulant arm [see creative funding II…], but for unclear reasons, that didn’t happen.
As I said, I thought their reporting was balanced and useful. My only complaints were that they used Extended-Release Guanfacine [Intuniv] and that Dr. Cahill is an adviser to Shire – but that’s no small complaint. The introduction of Extended Release products as a way of extending patents and profits is well known [Paxil, Seroquel, etc] and I think it’s "dirty pool" unless they have solid evidence that it’s better than the original [and they never do]. Likewise, this is a publicly funded study keyed to a commercial product [when there’s no reason to pick the ER product]. Either let Shire foot the bill, or do Guanfacine for Hyperactivity in Children With Autism Spectrum Disorder study. If the why? isn’t obvious, take a long look at this:
| Walmart Pharmacy [with goodRx card] | ||||||||
| drug | size | price | quanity | unit cost | ||||
| Tenex [Brand] | 1mg | $79.77 | 30 | $2.66 | ||||
| Guanfacine | 1mg | $4.00 | 30 | $0.13 | ||||
| Intuniv® | 2mg | $200.23 | 30 | $6.67 | ||||
| Guanfacine ER § | 2mg | $31.62 | 30 | $1.05 | ||||
Even if Shire had funded it, all Extended Release extend-the-patent studies should require the non-extended-release drug as a camparator. Except for that point [again, a big point], I think the study, particularly as originally described, was deserving of its public funding. As for Dr. Emslie’s editorial, he’s is a high volume KOL whose name is all over the industry-funded C&A RCTS. In the commentary, he encourages more independently funded RCTs to look for more than efficacy, actually a reasonable point.
If not found to be either safe or effective, the results of this proposed trial would also be highly informative given the significant proportion of TRD patients who, despite the relative paucity of data from independently-funded studies of rigorous design, are prescribed atypical antipsychotic agents off-label"…
The number needed to treat for ziprasidone was 7, which is similar to the other atypicals; however, the 95% confidence interval for the number needed to treat in this sample of 139 patients is very broad. As a result, we should be very cautious about efficacy comparisons. The relatively high rate of discontinuation due to adverse events is similar to the rate for the 300 mg/day dosage of extended-release quetiapine. As with quetiapine, somnolence is the most frequent side effect of ziprasidone. In this study, 34% of patients in the ziprasidone group experienced somnolence or fatigue… On other parameters, the rate of akathisia with ziprasidone was lower than with aripiprazole but greater than with quetiapine or olanzapine. Weight gain was less with ziprasidone than with olanzapine but greater than with aripiprazole or quetiapine. The present study suggests that adjunctive ziprasidone is effective in major depression, but it appears to have a relatively high rate of discontinuation due to adverse events and a high level of somnolence compared with other atypicals…
And as for medication cost:
| Walmart [with goodRx card] | ||||||||
| drug | size | price | quanity | unit cost | ||||
| Geodon® | 60mg | $983.42 | 60 | $16.39 | ||||
| Ziprasidone § | 60mg | $118.96 | 60 | $ 1.98 | ||||
Interestingly, the first author on this paper [George I. Papakostas] and the author of the commentary [J. Craig Nelson] were co-authors of a 2009 meta-analysis of the Atypical Antipsychotics as augmentation for Treatment Resistant Depression [Atypical Antipsychotic Augmentation in Major Depressive Disorder: A Meta-Analysis of Placebo-Controlled Randomized Trials – full text]. This figure is adapted from that meta-analysis [the NNT from this Geodon® study was 6.8 and the Odds Ratio was 1.7]:
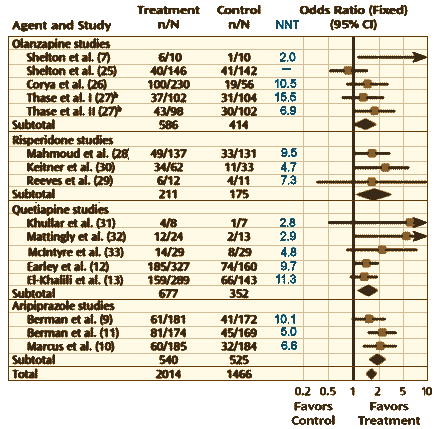
There have been three additional Aripiprazole Studies since that meta-analysis [I couldn’t extract the values from the complicated Fava et al crossover study]. In addition, there are two recent positive studies for brexpiprazole [cloned studies] [for an Abilify clone] recently approved by the FDA for augmentation in TRD [full text here and here]:
| Additional Aripiprazole Studies | ||||||||||
| Study | Treatment | Control | NNT | OR | 95% CI | |||||
| Lenze et al. § | 40/91 | 26/90 | 6.6 | 2.0 | 1.1-3.7 | |||||
| Kamijima et al | 159/392 | 55/195 | 8.1 | 1.4 | – | |||||
| Fava et al | essentially a negative study [crossover] | |||||||||
So back to the main thread here. I was disappointed that the NIMH had funded the Scahill et al study of Guanfacine in Autism Spectrum Disorder with the in-patent Extended Release form of the drug rather than the generic. It struck me as an ad for Shire’s product. I’m currently of the mind that it’s time for us to stop allowing PHARMA to use our journal articles for any commercial purposes. The article, however, was useful so I would’ve settled for just a comment about the funding somewhere. But with the Geodon article, I can see no reason for the NIMH to fund that study period. Its only purpose is to say, "Me too. My drug augments TRD too!" And with as much literature as we already have on this point, who actually needs to hear that? So I didn’t like the NIMH funding it nor the American Journal of Psychiatry publishing it. Send it to the Journal of Clinical Psychiatry [and let Pfizer pay for it]. We’ve had enough of that use of the scientific literature in psychiatry for this whole century already.
The history of so-called atypical antipsychotic (AAP) drugs for treatment resistant depression reflects badly on the field. One could see the writing on the wall already by the mid-1990s – indication creep for the AAP drugs progressed from the original use in schizophrenia to mania then to bipolar depression and finally to the biggest prize of all – unipolar depression. Make that nonpsychotic unipolar depression. Was there ever an a priori pharmacological rationale for their use in nonpsychotic primary mood disorders? Not really – just a lot of hand waving by KOLs jumping on the bandwagon.
I am on record with critiques of claims for risperidone and aripiprazole as augmenting agents for TRD. The problems are as Dr. Mickey describes them – a focus on nominal efficacy against placebo rather on effectiveness (their NNTs are weak); absence of evidence for long term benefit; glossing over of surprisingly common side effects such as tardive dyskinesia and akathisia, not to mention metabolic side effects; and studious avoidance of comparative efficacy trials against established augmenting agents such as lithium. Those are all typical features of experimercials as distinct from dispassionate clinical trials. When such studies are combined into an uncritical meta-analysis like the Nelson-Papakostas report in American Journal of Psychiatry mentioned above by Dr. Mickey, then clinicians mistakenly think they have a green light to use the AAP drugs liberally for nonpsychotic depressed patients. AAPs are not Pez, as I pointed out in AJP in response to the Nelson-Papakostas report.
Dear Mickey, I’m a bit puzzled by your being so generous with Dr. Emslie. I could not read his editorial or the Scahill article without associating both to S329. Emslie, of course, was one of the S329 authors. And, in my mind, the Scahill paper bears an eerie resemblance to S329: We once again have a gaggle of academic authors with doctoral degrees, 3 of whom acknowledge COI’s, 2 of them specifically with Shire. The conclusion also sounds terribly familiar: “Extended release guanfacine appears to be safe and effective….” Heard that one before.
However, there is in fact at least one major difference: The listed authors in the current article thank 5 non-doctoral degreed persons [the cited degrees are BA’s, an MS, and an MBA, while 2 people go without any degrees mentioned]. The thanks are for performing tasks such as data management, study coordination and assistance with manuscript preparation. Now there is a difference–I do not recall such a courtesy being extended to the non-doctors who, in the end, turned out to be the actual [ghost] writers and for hire data jugglers involved in the publication of S329.