by J. Craig Nelson, M.D. and George I. Papakostas, M.D.American Journal of Psychiatry. 2009 166:980-991., September 2009
Objective: The authors sought to determine by meta-analysis the efficacy and tolerability of adjunctive atypical antipsychotic agents in major depressive disorder.Method: Searches were conducted of MEDLINE/PubMed [1966 to January 2009], the Cochrane database, abstracts of major psychiatric meetings since 2000, and online trial registries. Manufacturers of atypical antipsychotic agents without online registries were contacted. Trials selected were acute-phase, parallel-group, double-blind controlled trials with random assignment to adjunctive atypical antipsychotic or placebo. Patients had nonpsychotic unipolar major depressive disorder that was resistant to prior antidepressant treatment. Response, remission, and discontinuation rates were either reported or obtained. Data were extracted by one author and checked by the second. Data included study design, number of patients, patient characteristics, methods of establishing treatment resistance, drug doses, duration of the adjunctive trial, depression scale used, response and remission rates, and discontinuation rates for any reason or for adverse events.Results: Sixteen trials with 3,480 patients were pooled using a fixed-effects meta-analysis. Adjunctive atypical antipsychotics were significantly more effective than placebo [response: odds ratio=1.69, 95% CI=1.46–1.95, z=7.00, N=16, p<0.00001; remission: odds ratio=2.00, 95% CI=1.69–2.37, z=8.03, N=16, p<0.00001]. Mean odds ratios did not differ among the atypical agents and were not affected by trial duration or method of establishing treatment resistance. Discontinuation rates for adverse events were higher for atypical agents than for placebo [odds ratio=3.91, 95% CI=2.68–5.72, z=7.05, N=15, p<0.00001].Conclusions: Atypical antipsychotics are effective augmentation agents in major depressive disorder but are associated with an increased risk of discontinuation due to adverse events.
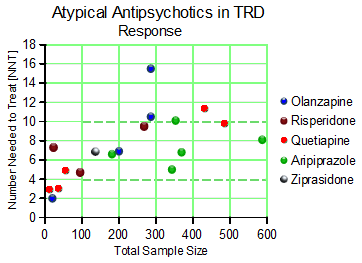
For reference, in a treatment situation like this, an NNT < 4.0 would be a respectable effect size [TCAs are in the range of 3]. An NNT > 10 would be considered no effect at all. As you can see, for the large studies [n > 100], they range from 5.0 to 15.5, average 8.9. That’s a weak effect for this situation. It means treating ~nine cases to beat placebo in one.
While the efficacy of the atypical agents for adjunctive therapy in major depressive disorder appears fairly well established, there are other considerations. The rate of discontinuation due to adverse events was significantly higher for the atypical group [9.1%] than the placebo group [2.3%]. The risk difference by meta-analysis was 0.06, with a number needed to harm of 17. While the discontinuation rates did not differ significantly among the agents, rates of specific side effects may be quite different. In addition, during continuing treatment there may be other secondary effects that in the aggregate affect tolerability and patient acceptance. The atypical agents also are associated with a variety of relatively serious adverse effects, such as metabolic syndrome, extrapyramidal symptoms, and rare but serious symptoms such as tardive dyskinesia and neuroleptic malignant syndrome. As a consequence, the risk-benefit ratio appears to be different from that of several alternative treatments for major depressive disorder.
And in today’s world, there are always drug prices to keep in mind [this is the current cost/pill from goodRx with their discount coupon paying cash @ Walmart] [users of insurance are paying more] [the dates may be slightly off because of my patent-exclusivity-dyslexia, but they’re close]:
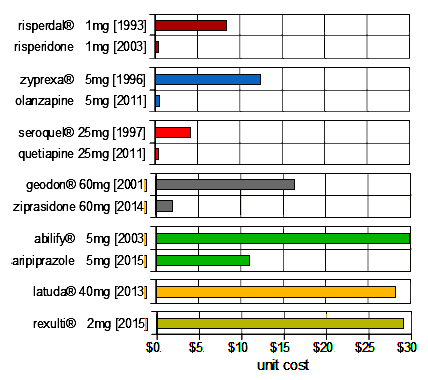
Let me say that again – "this is the current cost/pill" and a low-ball version at that [for the Brand® drugs, this is today’s cost as I couldn’t find their cost when introduced]. There aren’t many people around to whom this wouldn’t matter – at least not where I live. The escalation in price for the Brand drugs is well beyond inflation. The cost for the generic looks to be moving skyward. Unless you’re using one of the generics from the 1990s’ drugs, you’re quickly into sticker-shock territory.
A couple of other things. I’m not excited about adding a withdrawal-prone drug to another withdrawal-prone drug. I’ve had several cases where getting somebody off the combination is something of a nightmare. There’s another thing I don’t really understand. In the past, we saw depression as time limited. If a person responded to antidepressants, we usually continued them for 6 months to prevent relapse. But except in cases of patients with recurrent episodes of depression, we stopped the drugs after a time. These days, people stay on medications forever. At first, I thought it was what their doctors were telling them, but the patients themselves are reticent to stop. For some, it’s a misinterpretation of withdrawal. But for many, they are reluctant to stop them for no apparent reason. I presume they’ve got the idea that they are treating or protecting against some depressive disease. So I’ve taken to saying that the drugs aren’t for-ever, they’re for-a-while. up front. And these days, those responding patients who just won’t stop are in my mind every time I write a prescription.
Prices like $29 per pill for brexpiprazole (Rexulti) and $28 per pill for lurasidone (Latuda) give us more than sticker shock – they give us moral outrage. It’s worse than a Martin Shkreli déjà vu because these drugs are going to be indicated for a much longer period of treatment (months) than pyrimethamine (Daraprim) needs to be used (2-3 weeks for treatment of toxoplasmosis and just 2 days for treatment of malaria).
And where are the comparative effectiveness data to justify those prices for 2 new, me-too atypical antipsychotic drugs? These corporations are thumbing their noses at us just like Shkreli does – they do it just because they can get away with it. It really is time that this laissez-faire market was reined in. A good place to start would be with a regulatory searchlight on marketing expense disguised as R&D expense.
While I also have great concerns about using these drugs, I keep asking myself whether outcomes are affected by poor diagnostic criteria. This may be another case of pharma shooting themselves in the foot.
See Consumer Reports November 2011 Should you take an antipsychotic drug to treat depression? http://www.consumerreports.org/cro/2011/11/should-you-take-an-antipsychotic-drug-to-treat-depression/index.htm
Editor’s Note:
These materials are made possible by a grant from the state Attorney General Consumer and Prescriber Education Grant Program, which is funded by the multi-state settlement of consumer-fraud claims regarding the marketing of the prescription drug Neurontin (gabapentin).
And here’s another from December 2013 http://www.consumerreports.org/cro/2013/12/treating-anxiety-adhd-depression-insomnia-and-ptsd-with-newer-antipsychotics/index.htm
http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001403
Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC (2013) Adjunctive Atypical Antipsychotic Treatment for Major Depressive Disorder: A Meta-Analysis of Depression, Quality of Life, and Safety Outcomes. PLoS Med 10(3): e1001403. doi:10.1371/journal.pmed.1001403
Thanks to Altostrata above. I was going to suggest that your readers would benefit from reading my team’s more in-depth meta-analysis of atypical antipsychotics for depression. We included data from more sources and used more efficacy and safety outcomes. Our conclusions were less positive than those reached by Nelson & Papakostas. We also included several critiques about the design of these trials.
Thanks Glen,
I’m actually working on a discussion of your meta-analysis vis-à-vis Nelson and Papakostas as we speak. That’s the why of my “stats” posts these last couple of days. I’m interested in the funnel plot, and some of their forest plots that don’t feel quite right, so I’m reading some of their references. I hadn’t seen yours until Alto mentioned it. As always, yours is a gem [but lots of stuff]…
Mickey,
We explicitly discuss the differences between our analysis and Nelson/Papakostas from the bottom of page 20 through part of page 21 in our paper.
One more quick thought: How does one conduct a truly double-blind trial of antipsychotic augmentation for MDD? The side effect difference between Seroquel, Abilify, Symbyax, or Risperdal versus a placebo is going to look pretty obvious to both clinical raters and patients.
I will be interested to see what you have to say about these meta-analyses.
Mickey, just came upon your site. I am enjoying catching up on some favorite topics and learning about new ones.
I wonder if you have followed the recent FDA decision to approve flibanserin (AddyI) for female “hypoactive sexual desire disorder”? Invented disease, toxic drug, hired experts, fake social media outcry, safety tests in men: what possible concerns could anyone have?
You, Otsuka and Lundbeck have convinced me to put together a presentation on antipsychotics and depression. I will let you know how it goes. Best wishes, Daniel.