Committee on Strategies for Responsible Sharing of Clinical Trial DataINSTITUTE OF MEDICINE OF THE NATIONAL ACADEMIES, pp. 290, 2015[for reference]
PLOS MedicineNational Library of Medicine, National Institutes of Healthby Deborah A. Zarin and Tony TseJanuary 19, 2016
-
Index of Suspicion: The tone of the IOM Report and the article is very civil with talk of Data Sharing for other novel investigations, limiting the access by levels depending on what’s being looked for, using the ClinicalTrials.gov Results Database. etc.We don’t want Data Transparency to look for novel anything. We want Data Transparency because it has been tampered with and distorted on a massive scale. We don’t want limited anything. We want to be able to look in every last nook and cranny. So I had a strong "con job" feeling [really strong].
-
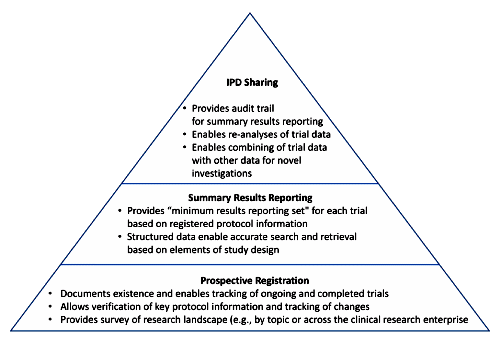
CinicalTrials.gov: I love this site – visit it all the time – pour through the history of changes fequently. But it’s no panacea for Data Transparency. It describes the study, but it doesn’t have the a priori Protocol, and it doesn’t have the Statistical Analytic Plan, and it’s obviously easy to leave out important details. If the study is being done by a CRO, it diesn’t say which one. So it’s great in a general way, but specifics, not so much.
-
CinicalTrials.gov Results Database: This is the most ignored requirement on the planet. The articles documenting how rarely it’s actually filled out are everywhere. It’s ignored by Industry and Academia alike. It’s improved some after all the attention its disuse gathered, but is hardly a reliable recource. And even if it is filled our, it rarely adds much to the published article. It’s as easy to jury-rig as the articles, maybe moreso because to my knowledge, it’s not reviewed. I hope the IOM Report will address this point.
-
The Horse’s Mouth: CinicalTrials.gov and the CinicalTrials.gov Results Database are secondary proxies for the actual data, and written by the sponsors. The other resource that are available are the FDA Medical Reports on Drugs@FDA. They’re also great, though often, the lag time can be excessive [the last one I went after took 18 months]. But all of these things are about the data, not the data itself. Even the FDA Medical Reports are Drugs@FDA. They’re too far from The Horse’s Mouth.
-
Viva Europe!: The verdict is still out on how the EMA is going to deal with the IPD information, but they’re off to a good start so far. What they’re releasing with some CCI redactions is exactly what was sent to them in the application for approval. That’s going to be the Drug Company on it’s best behavior, the place where they’re least likely to do a lot of spinning because it’s going to be evaluated by experts who have the power to ask for more information. So it’s the highest level surrogate information available. As someone checking it over, you’re at an equal level with the regulators. What we can get from the FDA is what they said about what they saw. What we will be able to get from the EMA is also what they saw. Much better…
COMMITTEE ON STRATEGIES FOR RESPONSIBLE SHARING OF CLINICAL TRIAL DATA
BERNARD LO (Chair), President, The Greenwall FoundationTIMOTHY COETZEE, Chief Research Officer, National Multiple Sclerosis SocietyDAVID L. DeMETS, Professor and Chair, Department of Biostatistics and Medical Informatics, University of Wisconsin–MadisonJEFFREY DRAZEN, Editor-in-Chief, New England Journal of MedicineSTEVEN N. GOODMAN, Professor, Medicine & Health Research & Policy, Stanford University School of MedicinePATRICIA A. KING, Carmack Waterhouse Professor of Law, Medicine, Ethics and Public Policy, Georgetown University Law CenterTRUDIE LANG, Principal Investigator, Global Health Network, Nuffield Department of Medicine, University of OxfordDEVEN McGRAW, Partner, Healthcare Practice, Manatt, Phelps & Phillips, LLPELIZABETH NABEL, President, Brigham and Women’s HospitalARTI RAI, Elvin R. Latty Professor of Law, Duke University School of LawIDA SIM, Professor of Medicine and Co-Director of Biomedical Informatics of the Clinical and Translational Science Institute, University of California, San FranciscoSHARON TERRY, President and CEO, Genetic AllianceJOANNE WALDSTREICHER, Chief Medical Officer, Johnson & Johnson

by Dan L. Longo, and Jeffrey M. DrazenNew England Journal of Medicine. 2016 374:276-277.The aerial view of the concept of data sharing is beautiful. What could be better than having high-quality information carefully reexamined for the possibility that new nuggets of useful data are lying there, previously unseen? The potential for leveraging existing results for even more benefit pays appropriate increased tribute to the patients who put themselves at risk to generate the data. The moral imperative to honor their collective sacrifice is the trump card that takes this trick…
A second concern held by some is that a new class of research person will emerge — people who had nothing to do with the design and execution of the study but use another group’s data for their own ends, possibly stealing from the research productivity planned by the data gatherers, or even use the data to try to disprove what the original investigators had posited. There is concern among some front-line researchers that the system will be taken over by what some researchers have characterized as “research parasites”…

Sorry, the comment form is closed at this time.