-
2001:
Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomized, Controlled Trial. "Paroxetine is generally well tolerated and effective for major depression in adolescents." -
2002: Fluoxetine for Acute Treatment of Depression in Children and Adolescents: A Placebo-Controlled, Randomized Clinical Trial. "Fluoxetine was superior to placebo in the acute phase treatment of major depressive disorder in child and adolescent outpatients with severe, persistent depression."
-
2003: Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. "…sertraline is an effective and well-tolerated short-term treatment for children and adolescents with MDD."
-
2004:
A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. "…treatment with citalopram reduced depressive symptoms to a significantly greater extent than placebo treatment and was well tolerated."
by Wagner KD, Ambrosini P, Rynn M, Wohlberg C, Yang R, Greenbaum MS, Childress A, Donnelly C, Deas D; and the Sertraline Pediatric Depression Study GroupJournal of the American Medical Association. 2003, 290[8]:1033-41.
CONTEXT: The efficacy, safety, and tolerability of selective serotonin reuptake inhibitors [SSRIs] in the treatment of adults with major depressive disorder [MDD] are well established. Comparatively few data are available on the effects of SSRIs in depressed children and adolescents.OBJECTIVE: To evaluate the efficacy and safety of sertraline compared with placebo in treatment of pediatric patients with MDD.DESIGN AND SETTING: Two multicenter randomized, double-blind, placebo-controlled trials were conducted at 53 hospital, general practice, and academic centers in the United States, India, Canada, Costa Rica, and Mexico between December 1999 and May 2001 and were pooled a priori.PARTICIPANTS: Three hundred seventy-six children and adolescents aged 6 to 17 years with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-defined MDD of at least moderate severity.INTERVENTION: Patients were randomly assigned to receive a flexible dosage [50-200 mg/d] of sertraline [n = 189] or matching placebo tablets [n = 187] for 10 weeks.MAIN OUTCOME MEASURES: Change from baseline in the Children’s Depression Rating Scale-Revised [CDRS-R] Best Description of Child total score and reported adverse events.RESULTS: Sertraline-treated patients experienced statistically significantly greater improvement than placebo patients on the CDRS-R total score [mean change at week 10, -30.24 vs -25.83, respectively; P=.001; overall mean change, -22.84 vs -20.19, respectively; P=.007]. Based on a 40% decrease in the adjusted CDRS-R total score at study end point, 69% of sertraline-treated patients compared with 59% of placebo patients were considered responders [P=.05]. Sertraline treatment was generally well tolerated. Seventeen sertraline-treated patients [9%] and 5 placebo patients [3%] prematurely discontinued the study because of adverse events. Adverse events that occurred in at least 5% of sertraline-treated patients and with an incidence of at least twice that in placebo patients included diarrhea, vomiting, anorexia, and agitation.CONCLUSION: The results of this pooled analysis demonstrate that sertraline is an effective and well-tolerated short-term treatment for children and adolescents with MDD.
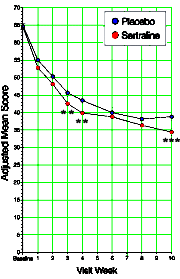 Figure 2. Weekly and Overall Adjusted Mean CDRS-R Scores
Figure 2. Weekly and Overall Adjusted Mean CDRS-R ScoresCDRS-R indicates Children’s Depression Rating Scale—Revised Best Description of Child total score. Data are least square means at each visit week, with mean scores averaged to give the overall mean, from a repeated-measures mixed-model analysis with age category, site, treatment, week, and week-by-treatment interaction used as fixed effects, subject as a random effect, and baseline effect as a covariate. Error bars indicate SE of the adjusted means, derived from the repeated-measures mixed-model procedure. P values are as follows: week 1. P=.09; week 2, P=.08; week 3, P =.01; week 4, P=.008; week 6, P=.37; week 8, P=.18; week 10, P=.001; and mean response, P=.007.
Right out of the gate, we have good reason to question these conclusions just by looking at the primary outcome variable graph which only achieved significance at 3, 4, and 10 weeks. But there’s a lot more to give us pause. In all of the depositions, Wagner makes it clear that she neither saw all the data nor checked the analysis in this study either, and that it was written at Pfizer with her coming in as the drafts were revised. Pfizer has gotten off light in the critiques of these clinical trials in that they had a writing firm, Current Medical Directions, that ghost wrote their Zoloft articles faster than they could find KOLs to sign up as guest authors [see zoloft: beyond the approval I… for this part of the story].
To the Editor: Dr Wagner and colleagues reported that sertraline was more effective than placebo for treating children with major depressive disorder and that it had few adverse effects. As one of the study group investigators in this trial, I am concerned about the way the authors pooled the data from 2 trials, a concern that was raised by previous letters critiquing this study. The pooled data from these 2 trials found a statistically marginal effect of medication that seems unlikely to be clinically meaningful in terms of risk and benefit balance.
New information about these trials has since become available. The recent review of pediatric antidepressant trials by a British regulatory agency includes the separate analysis of these 2 trials. This analysis found that the 2 individual trials, each of a good size [almost 190 patients], did not demonstrate the effectiveness of sertraline in treating major depressive disorder in children and adolescents.
E. Jane Garland, MD, FRCPC
Department of Psychiatry
University of British Columbia
VancouverCommittee on Safety of Medicines. Medicines and Health Care Products Regulatory Agency. Selective Serotonin Reuptake Inhibitors [SSRIs] — overview of regulatory status and CSM advice relating to major depressive disorder [MDD] in children and adolescents: summary of clinical trials. Available at: http://medicines.mhra.gov.uk/ourwork/monitorsafequalmed/safetymessages/ssrioverviewclintnaldata_101203.htm. Accessibility verified March 12, 2004.
Reply: In response to Dr Garland, our combined analysis was defined a priori, well before the last participant was entered into the study and before the study was unblinded. The decision to present the combined analysis as a primary analysis and study report was made based on …
-
2001:
Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomized, Controlled Trial. "Paroxetine is generally well tolerated and effective for major depression in adolescents." -
2002: Fluoxetine for Acute Treatment of Depression in Children and Adolescents: A Placebo-Controlled, Randomized Clinical Trial. "Fluoxetine was superior to placebo in the acute phase treatment of major depressive disorder in child and adolescent outpatients with severe, persistent depression."
-
2003:
Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. "…sertraline is an effective and well-tolerated short-term treatment for children and adolescents with MDD." -
2004:
A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. "…treatment with citalopram reduced depressive symptoms to a significantly greater extent than placebo treatment and was well tolerated."
Mickey,
Thanks for keeping on. I’ve written before about the similarities between psychiatry shenanigans and diabetes shenanigans (how the synthetic ‘insulin’ became the gold standard. Thought you might enjoy the following article, illustrating just how far-reaching jury-rigging goes.
A popular weight-loss pill was buoyed by studies that understated its harms at
https://www.statnews.com/2016/08/16/weight-loss-pill-harms/