I had assumed that the version of the Biederman documents I was using was all that was available. That was wrong as EP pointed out. psychrights had the complete release. When I located it before, I thought it was ‘broken,’ not realizing that it was just a huge file that took its time traveling to Georgia [14+ Mb].
Here is a more complete piece of the RISPERDAL 2002 Business Plan Summary added as an update to the end of the last post:
The clinical development program for RISPERDAL has yielded important new efficacy and safety data in the child and adolescent area. These efforts have previously been focused in the area of Disruptive Behavior Disorders and Subaverage IQ. Several trials, RI5-U5A-93 and RI5-CAN- 19 (as well as the open label 48 week follow up trials, USA-97 and CAN-20), initially designed to support filing for an FDA indication, have been completed and have yielded an impressive volume of new efficacy and safety data. Unfortunately, the FDA determined that Disruptive Behavior Disorder lacks the diagnostic specificity necessary to receive an approved indication. Nevertheless, these studies have contributed significantly to the clinical knowledge of RISPERDAL in the child and adolescent population, and provide a basis for ongoing medical education activities. Going forward, regulatory and clinical development efforts will include the evaluation of the NIMH RUPP Risperidone in Autism database to support filing for potential autism indication, adolescent schizophrenia indication, and the fulfillment of the FDA Written Request requirement for additional 6 month patent exclusivity. It is expected that the request will include the following requirements:
• Pediatric PK trial;
• Adolescent schizophrenia trial; and
• Pediatric bipolar trial.
The child and adolescent market offers several unique challenges not found in other areas where RlSPERDAL is used. First and foremost, the sensitivity towards medications and their use in children shapes this segment. This Issue is prominent in medical as well as lay press and public discussions regarding pediatric psychopharmacology. This results in many stereotypes and stigmas that prevent some children from receiving appropriate treatment. Additionally, the child and adolescent market is not driven by diagnosis, but rather by treatment of symptoms such as aggression, agitation, self-injurious behavior, and explosive rage. This lack of consistent diagnosis stems from a reluctance to "label" children at an early age, as well as a fundamental lack of consensus regarding the actual underlying disease states causing this symptomatic behavior. As a result, multiple diagnoses and comorbidities are the rule, rather than the exception in this area. These issues have influenced the clinical development process and limited the ability to achieve an FDA approved indication for RISPERDAL in children…
"… many stereotypes and stigmas that prevent some children from receiving appropriate treatment." I’ve never been in the business world and know nothing of marketing, but I expect this is pretty much standard fare – knowing your market or something like that. If they were discussing washday detergents or toothpaste, it would sound much the same and it wouldn’t bother anybody. But they’re not. They’re talking about big drugs for small children, so it takes on another dimension. And we probably wince at the statement that these "stereotypes and stigmas" are interfering with "appropriate treatment." Who are they to define what is appropriate treatment?
"… the child and adolescent market is not driven by diagnosis, but rather by treatment of symptoms such as aggression, agitation, self-injurious behavior, and explosive rage" actually seems an accurate description of prescribing practices to me. It comes from their marketing research which they show in a slide that goes with their Business Plan:
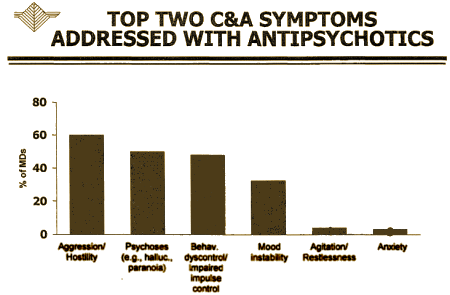
It’s no wonder that they started where they did: "These efforts have previously been focused in the area of Disruptive Behavior Disorders and Subaverage IQ. Several trials … initially designed to support filing for an FDA indication, have been completed…" It’s what their marketing research said to do.They seemed surprised the FDA didn’t approve it: "Unfortunately, the FDA determined that Disruptive Behavior Disorder lacks the diagnostic specificity necessary to receive an approved indication." It continues:
The bulk of RISPERDAL use in the child and adolescent market is for mood and anxiety related diagnoses [keeping in mind the limitation previously mentioned regarding diagnosis vs. symptomatic treatment]. Bipolar Disorder with 18% of uses leads the way in this area followed by Depression with 10% and Anxiety with 3%. This represents an overall mood and anxiety use of 31% of the total atypical drug use for children. ADHD/Conduct Disorder makes up the next largest market segment with 20% of total drug uses, followed by schizophrenia/psychosis at 15%, and autism at 14%. These usage patterns are consistent with those for all atypical antipsychotics in aggregate.
Reading this business plan, you can see what’s coming. They wanted to market their drug to Children and Adolescents so they initially went for Disruptive Behavior Disorder, which is hardly a diagnosis – it’s more like a ‘complaint.’ How that one made it into the DSM-IV is beyond me. In the DSM-III, they moved from a ‘disease’ model to a ‘descriptive model’ – lists of symptoms. But Disruptive Behavior Disorder isn’t even that, because it doesn’t describe the symptoms of the patient [child]. It describes the symptoms of the teacher or parent. It’s about the ‘impact’ of the child on others [Disruptive]. The FDA is [wisely] not about to approve a medication as efficacious because someone other than the person taking the medicine has a decreased symptom burden. So even if the medication is being prescribed for "Aggression / Hostility" and "Behavior dyscontrol / Impaired impulse control" – that’s not an indication. It’s a medication usage that should be carefully considered in every individual case where its contemplated, and carefully monitored when used. Some think the medication should never be used in this way. Some use it way too much in this way. But wherever one falls on that spectrum, it’s never a routine matter.
So the marketing department takes a second look and sees: "Bipolar Disorder with 18% of uses leads the way in this area." Is it any wonder that they suddenly decided to respond to Dr. Biederman’s requests that they fund a Center at Harvard/Mass General? Dr. Biederman was the primo Bipolar Child guru who had already given bipolar disorder in children a dose of steroids and was already an advocate for treating them with Atypical Antipsychotics. No one would question giving the medication for a disease. Why they could even recycle their earlier studies under the new label ["Several trials, RI5-U5A-93 and RI5-CAN- 19 (as well as the open label 48 week follow up trials, USA-97 and CAN-20), initially designed to support filing for an FDA indication, have been completed and have yielded an impressive volume of new efficacy and safety data"]. All they had to do was reanalyze the data and get Dr. Biederman to present it for them, and then later publish it [trial 93: a bad penny…]. And he did it!
In the deposition, the lawyer takes Dr. Biederman apart on the topic of this study [the one I called the bad penny]. Biederman didn’t admit it was ghost-written, but he might as well have. The paper claimed that Risperidone was effective on the Affective Symptoms [disruptive retarded kids]. As it turned out, the abstract mis-stated the results. It was actually more effective on the Non-Affective Symptoms!
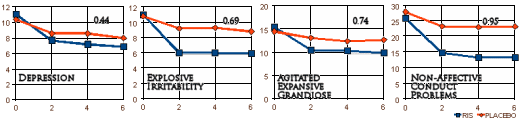
Here’s the lawyer pointing out the error:

But if you think about it, what does the result of a Risperidone Trial in disruptive retarded kids have to do with bipolar disorder in children, even if it had shown what they claimed [which it didn’t]? Stay tuned…



My layman’s reading of the clinical trial results of the Risperdal trials in children can be summed up as “Risperdal sedates mentally retarded children better than placebo”
No great mystery there…….No meaningful guidance either…..
Don’t usually have much of an opportunity to say this: “You go, lawyer!!!”