The story of Alan Schatzberg, Mifepristone, Stanford Medical School, and Corcept described below [Mifepristone 0: $tanford’s $chatzberg redux…] is a multi-layered saga hardly covered by the NIMH’s decision to scrap a study in 2009 – "but we’re done with the mifeprestone aspect." By now, we’re used to stories where the Pharmaceutical Industry wrapped its fingers around Academic Medicine pursuing "blockbuster sales." This tale is different because the corrupting fingers were on the hand of Academic Medicine itself. Freud’s analogy to ‘peeling an onion‘ seems to fit here, so I’ll start on the outside with the illness in question [Psychotic Depression] and the published attempts to treat it with Mifepristone. I guess this is chapter one of a series trying to parse a tangled story. The point is what happened when a simple academic question got contaminated from the start by the "rush to market."
Psychotic Depression is a big deal disease – a show stopper. It’s hard to even be around such patients – their melancholy is infectious. They are distant, somber, unable to discuss anything outside of their gloom. While they may be anxious or flat, over-eating or anorexic, sleeping too much or insomniac, pleading or mute – these parameters, like their mood, are unaffected by the environment. The "psychotic" parts are usually mood congruent breaks with reality ["I’m rotting inside" "I deserve to die for what I’ve done" "My mother calls me from the gave"] – less bizarre than the delusions of Schizophrenia but equally devastating. Even the most committed psychotherapist will usually cut short an exploratory interview, dropping back to a biological psychiatrist mode. This is no "talking disease." Because of the suicidal potential, such patients are usually hospitalized. They’re often resistant to antidepressants of any flavor, and adding antipsychotics, though sometimes helpful, can’t be counted on. Many end up being treated with electroconvulsive therapy [aesthetics aside, ECT passes the tests for an acceptable medical intervention – it is both safe and effective].
There are some simple observations that lead to the idea that Mifepristone might help in Psychotic Depression. Corticosteroids used to treat other diseases can cause a syndrome indistinguishable from naturally occurring Psychotic Depression. Corticosteroid production appears to be elevated in Psychotic Depression. Mifepristone is a steroid antagonist that blocks Progesterone at low doses [RU-486 – the "morning after" abortifactant], and blocks Cortisol at higher doses. So it makes sense to see what might happen if Mifepristone given taken by Psychotically Depressed patients. There are some problems over and above the fact that drug is controversial because of the abortion debates. Psychotically Depressed people aren’t just out there reading newspaper ads waiting to be treated. One would need to treat them when they show up, and they are in no state of mind to be easily recruited.
The drug patent for Mifepristone was obtained by Stanford and released to a private company in 1998, Corcept, founded and directed by Alan Schatzberg, the Chairman of the Stanford Department of Psychiatry. In 2001, Corcept initiated double-blind multi-center clinical trials of Mifepristone [CORLUX®] in Psychotic Depression. The first publication came out in 2001 as a Brief Report funded as a part of a larger NIMH Grant to Stanford with Dr. Schatzberg as the Principal Investigator.
|
Journal of Clinical Psychopharmacology by Belanoff JK, Flores BH, Kalezhan M, Sund B, and Schatzberg AF Department of Psychiatry, Stanford University Medical Center October 2001 |
|
The rationale for treating psychotic major depression with glucocorticoid receptor antagonists is reviewed. Five patients with psychotic major depression were given 600 mg of mifepristone in a 4-day, double-blind, placebo-controlled crossover study. All the patients completed the protocol and adverse effects were not observed or reported. All of the five patients showed substantial improvements in their Hamilton Rating Scale for Depression scores while they were receiving mifepristone, and four of the five patients showed substantial improvement in their Brief Psychiatric Rating Scale scores. Little, if any, improvement was seen with placebo. These preliminary results suggest that short-term use of GR antagonists may be effective in the treatment of psychotic major depression and that additional study, perhaps using higher doses or more treatment days, seems warranted.
|
The cases are well described in the published article – all hospitalized cases with clear evidence of Psychotic Depression. My read on the results differed from that of the authors. There was only one patient who lived up to the title ["Rapid reversal of psychotic depression…"]. Another case got better after a time with further treatment. A third case seemed to improve a bit though he remained significantly impaired. Two cases were treatment failures. I saw the published rating scales differently as well [my graphs derived from the published table]:
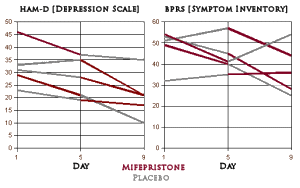
On the HAM-D scores, everyone did improve on Mifepristone as advertised, but except for one patient, it looks to me like being in the hospital itself helps depression rather than much of a drug effect. On the BPRS, the drug seemed to change the course of two of the subjects. I wouldn’t quibble that the data didn’t support further study, but I would sure question the title and I thought the reading of the objective data was overly optimistic.
The next study came out about a year later from Corcept:
|
Biological Psychiatry by Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu EE, Schold C, and Schatzberg AF. Corcept Therapeutics Inc., Menlo Park, California September 2002 |
|
BACKGROUND: The rationale for treating patients with psychotic major depression [PMD] with glucocorticosteroid receptor [GR] antagonists is explained.
METHODS: Thirty patients with PMD, with Hamilton Rating Scale for Depression [HAMD-21] scores of 18 or greater, were assigned in an open label trial to receive 50 mg, 600 mg, or 1200 mg of mifepristone for 7 days.
RESULTS: All the subjects completed the protocol; there were no dropouts. Side effects were mild and sporadic. Of 19 subjects in the combined 600- and 1200-mg group, 13 had a 30% or greater decline in their Brief Psychiatric Rating Scale [BPRS] scores, compared with 4 of 11 in the 50-mg group. In the 600- and 1200-mg group, 12 of 19 subjects showed a 50% decline in the BPRS positive symptom subscale, a more sensitive index for the symptoms seen in PMD, compared with 3 of 11 in the 50-mg group; 8 of 19 subjects in the 600- and 1200-mg group had a 50% decline in the HAMD-21, compared with 2 of 11 in the 50-mg group.
CONCLUSIONS: These results suggest that short term use of GR antagonists may be effective in the treatment of psychotic major depression and that further blinded studies are warranted.
|
This trial involved six University centers and thirty patients divided into three dose levels of Misepristone [a 60mg control goup, a 600mg group, and a 1200mg group] given for seven days, assessed using the same instruments as the first study [HAM-D and BPRS] [the data from a subscale of the BPRS felt to reflect Psychosis (POS) was included]. They don’t say these were inpatients, but I presumed they were based on a comment about daily observation. The authors again showed no statistical analysis. Their Day 0 and Day 7 raw data was available in tabular form. I plotted the %Change from Day 0 to Day 7 for the three indexes at the different dose levels [means in red].
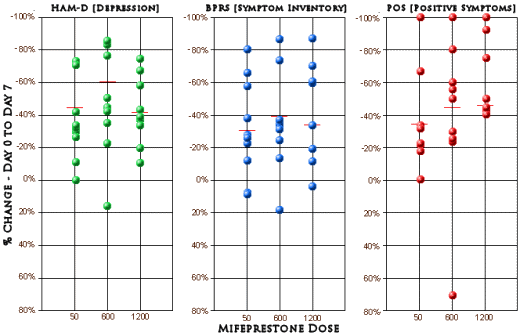
While others have done statistical analyses and found no significance, all I did was look at the plots. Frankly I don’t see much of anything that needs further analysis [those cut-off percentages in the reported results seem arbitrary and obscure the amount of obvious variance in the data]. I might’ve called it a bust at this point, but the authors seemed to think otherwise.
[…] Boring Old Man « Mifepristone I: the outer layer… Mifesterone II: the “wonder […]