
 Moving along. Trial 0008 was Zeneca‘s most successful trial for Seroquel, published in a more widely distributed journal. I’ve included the abstract in part because it describes the actual dosing schedule for Seroquel omitted from the F.D.A. statistical report.
Moving along. Trial 0008 was Zeneca‘s most successful trial for Seroquel, published in a more widely distributed journal. I’ve included the abstract in part because it describes the actual dosing schedule for Seroquel omitted from the F.D.A. statistical report. Quetiapine in patients with schizophrenia. A high- and low-dose double-blind comparison with placebo
Archives of General Psychiatry. 1997 Jun;54(6):549-57.
by Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CG.
Division of Mental Health, Larue D. Carter Memorial Hospital, Indianapolis, Ind.
AbstractBACKGROUND: Quetiapine fumarate (Seroquel [ICI 204,636]) is an atypical dibenzothiazepine antipsychotic with a greater affinity for 5-hydroxytryptamine2 (5-HT2) receptors than for D2 dopamine receptors; its efficacy in patients with schizophrenia was shown in early phase 2 trials (maximum dose, 750 mg/d).METHODS: In this multicenter, double-blind, placebo-controlled trial, 286 patients hospitalized with chronic or subchronic schizophrenia [DSM-III-R] were randomized to 6 weeks of treatment with high-dose quetiapine fumarate (< or = 750 mg/d), n = 96; low-dose quetiapine fumarate (< or = 250 mg/d), n = 94; or placebo, n = 96. The Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impression Severity of Illness item scores were the primary efficacy variables. Secondary efficacy variables included the BPRS positive-symptom cluster score, the Modified Scale for the Assessment of Negative Symptoms summary score (United States only), and the total score from the negative scale of the Positive and Negative Syndrome Scale (Europe only). Scores were analyzed using an analysis of covariance for change from baseline at end point with last observations carried forward. The model included baseline score (covariate), center, and treatment. Extrapyramidal symptoms were assessed using the Simpson-Angus Scale and the Barnes Akathisia Scale; abnormal involuntary movements were assessed using the Abnormal Involuntary Movement Scale. Frequency distributions of grouped change-from-baseline scores were analyzed using chi 2 tests.
RESULTS: Of 280 patients in whom the efficacy of quetiapine was evaluated, 159 (42% of those receiving high-dose treatment; 57%, low-dose treatment; and 59%, placebo) withdrew before trial completion, primarily because of treatment failure. Significant (P < .001, BPRS; P = .003, Clinical Global Impression Severity of Illness item; and P = .003, BPRS positive-symptom cluster) differences were identified between patients receiving high-dose quetiapine and placebo for both primary efficacy variables, with end point differences in the BPRS positive-symptom cluster score showing quetiapine’s consistency in reducing positive symptoms. The reduction of negative symptoms was less consistent; high-dose quetiapine was superior on the Modified Scale for the Assessment of Negative Symptoms but not on the negative scale of the Positive and Negative Syndrome Scale. Quetiapine was well tolerated and did not induce extrapyramidal symptoms, sustained elevations of prolactin, or clinically significant changes in hematologic parameters.
CONCLUSIONS: Quetiapine is an effective antipsychotic with a favorable safety profile. The optimum dose is probably greater than 250 mg/d.
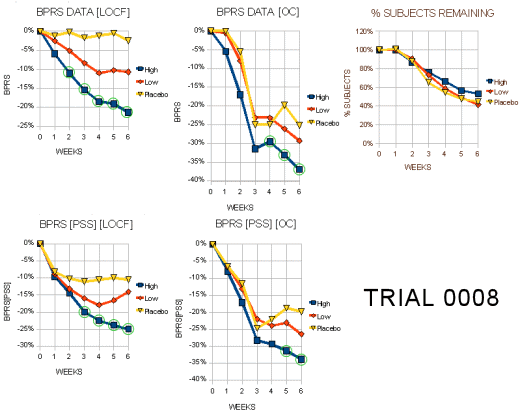

 I expect the people at Zeneca were pretty pleased to see those LOCF graphs on the left. That’s exactly what they hoped for. Even the OC graphs have the green circles indicating statistical significance [though they’re not quite so pretty]. Right now, we’re not talking about relevance so I’ll reserve any of those comments for a later time. Again, there were a lot of drop-outs. I’ve attempted to roughly translate the awkward bar graphs of the scores for the subjects that dropped out into a more readable form [the graph on the right is the inverse of the one above]:
I expect the people at Zeneca were pretty pleased to see those LOCF graphs on the left. That’s exactly what they hoped for. Even the OC graphs have the green circles indicating statistical significance [though they’re not quite so pretty]. Right now, we’re not talking about relevance so I’ll reserve any of those comments for a later time. Again, there were a lot of drop-outs. I’ve attempted to roughly translate the awkward bar graphs of the scores for the subjects that dropped out into a more readable form [the graph on the right is the inverse of the one above]: 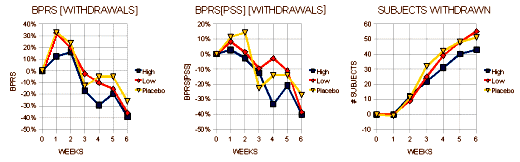
-
In the first few weeks, Seroquel appeared to allow the subjects to stay in the study, suggesting that it was helpful early on.
-
The High Dose Seroquel group had a 9% lower drop-out rate overall, again suggesting it was helpful.
Another F.D.A. report [Review and Evaluation of the Clinical Data] has a different version of the conclusion:

Sorry, the comment form is closed at this time.