You know, once you get into these drug industry matters, it just keeps on coming. Pharmalot, Soulful Sepulcher, Is Something Not Quite Right With Stan [hat tip to stan] are all over a recent article in PLoS Medicine about Abilify as a maintenance Bipolar therapy:
Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
by Alexander C. Tsai, Nicholas Z. Rosenlicht, Jon N. Jureidini, Peter I. Parry, Glen I. Spielmans, David Healy
PLoS Med 8(5):e1000434. doi:10.1371/journal.pmed.1000434
AbstractBackground: Aripiprazole, a second-generation antipsychotic medication, has been increasingly used in the maintenance treatment of bipolar disorder and received approval from the U.S. Food and Drug Administration for this indication in 2005. Given its widespread use, we sought to critically review the evidence supporting the use of aripiprazole in the maintenance treatment of bipolar disorder and examine how that evidence has been disseminated in the scientific literature.Methods and Findings: We systematically searched multiple databases to identify double-blind, randomized controlled trials of aripiprazole for the maintenance treatment of bipolar disorder while excluding other types of studies, such as open-label, acute, and adjunctive studies. We then used a citation search to identify articles that cited these trials and rated the quality of their citations. Our evidence search protocol identified only two publications, both describing the results of a single trial conducted by Keck et al., which met criteria for inclusion in this review. We describe four issues that limit the interpretation of that trial as supporting the use of aripiprazole for bipolar maintenance: (1) insufficient duration to demonstrate maintenance efficacy; (2) limited generalizability due to its enriched sample; (3) possible conflation of iatrogenic adverse effects of abrupt medication discontinuation with beneficial effects of treatment; and (4) a low overall completion rate. Our citation search protocol yielded 80 publications that cited the Keck et al. trial in discussing the use of aripiprazole for bipolar maintenance. Of these, only 24 (30%) mentioned adverse events reported and four (5%) mentioned study limitations.Conclusions: A single trial by Keck et al. represents the entirety of the literature on the use of aripiprazole for the maintenance treatment of bipolar disorder. Although careful review identifies four critical limitations to the trial’s interpretation and overall utility, the trial has been uncritically cited in the subsequent scientific literature.
They found that all roads recommending Abilify for this indication lead to only one clinical trial [A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania]. In analyzing that lonely little trial, they found a lot wrong with it [particularly (3) throwing their subjects into Abilify withdrawal]. But this is the part of that study that blew me away:
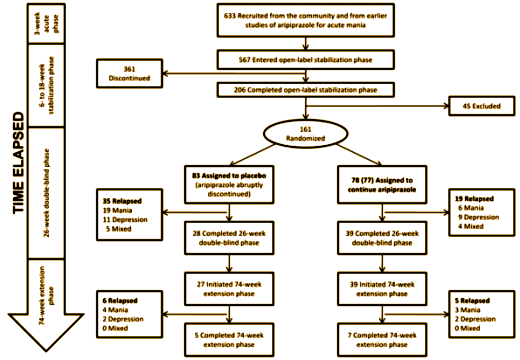
They went from 633 recruits down to 161 subjects who were stabilized on Abilify for 6-18 weeks. As you can see, more relapsed on placebo than Abilify [unless they were in Abilify withdrawal and that was confused with or precipitated relapse]. But look at the bottom box. They ended up with 12 subjects who went the distance. That’s 1.8% of the recruits. That’s 7.4% of those randomized. That’s 18% of the people who chose the last phase. Now that’s some serious dropping out! It’s hard to say it was a successful trial if 92% didn’t complete it. Even more impressive, of the 80 articles citing this study, only 4 mentioned its limitations. Once an article makes it into the literature, apparently the inertia of the printed word and abstract keeps the conclusions alive for a very long time…
Thank you for the link!