Fall is just getting going here in the mountains. Some cool mornings, the dogwood leaves are turning a dark red. It’s too soon to know if this is going to be one of those breathtakingly beautiful seasons or if it’ll be a race to brown. I can’t ever predict, but I spout theories anyway, "been a rainy summer so…" etc. I’m teaching a course every weekend for the month and am a little preoccupied with that. Fall visitors come and go. Cooking pork for the Fall Social this Saturday. But I’m in a kind of waiting mode. Waiting for what? The TMAP Trial in November, for one thing. And I wonder what’s going to happen in Australia with all of that medicating ‘Ultra High Risk’ patients. But I think the big picture is that I’m wanting something to happen to start putting a closing parentheses on what I consider to be a dark time in the history of psychiatry. It’s not like some election is going to happen and we will have a new administration [though that would be nice]. I don’t even know what it might be that would mark such a change. But I’m waiting for it never-the-less.
A Meta-Analysis of Data Submitted to the Food and Drug Administration
by Irving Kirsch, Brett J. Deacon, Tania B. Huedo-Medina, Alan Scoboria, Thomas J. Moore, Blair T. Johnson
PLoS Medicine 2008 5[2]:260-269.
[full text on-line]
Background: Meta-analyses of antidepressant medications have reported only modest benefits over placebo treatment, and when unpublished trial data are included, the benefit falls below accepted criteria for clinical significance. Yet, the efficacy of the antidepressants may also depend on the severity of initial depression scores. The purpose of this analysis is to establish the relation of baseline severity and antidepressant efficacy using a relevant dataset of published and unpublished clinical trials.
Methods and Findings: We obtained data on all clinical trials submitted to the US Food and Drug Administration [FDA] for the licensing of the four new-generation antidepressants for which full datasets were available. We then used meta-analytic techniques to assess linear and quadratic effects of initial severity on improvement scores for drug and placebo groups and on drug–placebo difference scores. Drug–placebo differences increased as a function of initial severity, rising from virtually no difference at moderate levels of initial depression to a relatively small difference for patients with very severe depression, reaching conventional criteria for clinical significance only for patients at the upper end of the very severely depressed category. Meta-regression analyses indicated that the relation of baseline severity and improvement was curvilinear in drug groups and showed a strong, negative linear component in placebo groups.
Conclusions: Drug–placebo differences in antidepressant efficacy increase as a function of baseline severity, but are relatively small even for severely depressed patients. The relationship between initial severity and antidepressant efficacy is attributable to decreased responsiveness to placebo among very severely depressed patients, rather than to increased responsiveness to medication.
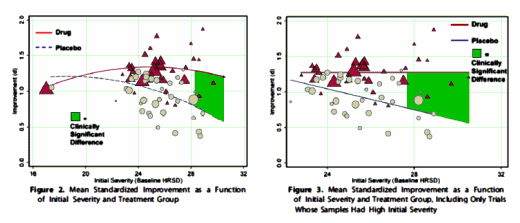
 In their meta-analysis, they looked at all the studies turned in to the FDA for the major antidepressants, not just the published studies. And they defined criteria for clinical significance rather than statistical significance. A quick look at the graphs [HAM-D Improvement vs HAM-D Baseline] shows the green Clinical Significance boxes sparsely populated, and that the changes were from a drooping Placebo curve, not a rising Drug curve [whatever that might mean]. This study is what many critics are referring to when they say "antidepressants don’t work except in severe depression." One of this month’s MedScape pieces sets out to debunk the debunking [un-debunk the debunking?]:
In their meta-analysis, they looked at all the studies turned in to the FDA for the major antidepressants, not just the published studies. And they defined criteria for clinical significance rather than statistical significance. A quick look at the graphs [HAM-D Improvement vs HAM-D Baseline] shows the green Clinical Significance boxes sparsely populated, and that the changes were from a drooping Placebo curve, not a rising Drug curve [whatever that might mean]. This study is what many critics are referring to when they say "antidepressants don’t work except in severe depression." One of this month’s MedScape pieces sets out to debunk the debunking [un-debunk the debunking?]: The floor effect: Or why it’s hard to show anything works in mild depression
MedScape
by Nassir Ghaemi, MD
September 26, 2011The debates about antidepressant controversy have involved many issues. One that has received little attention is the issue of a floor effect. In the most popularized review of antidepressant randomized clinical trial [RCTs] in the FDA database, the conclusion was that antidepressants are ineffective except in the most extreme depressive episodes. This was based on an analysis that for mild depression, antidepressants had almost the same effect as placebo. These comparisons are made using the Hamilton Depression Rating Scale [HDRS], a 17 item scale where 18 points is seen as the minimum score for a major depression, less than 24 is seen as mild depression, 24-28 is moderate, and above 28 is severe [with maximal score of 52]. In the above meta-analysis, the antidepressant group improved by 9.6 points from a baseline of 25.5. The placebo group improved by 7.8 points. The difference between the two groups of 1.8 points was seen as small, since the cut off for clinical significance was seen as 3 points. Hence the conclusion: antidepressants don’t work. When assessed by severity of baseline depression, those with severe depression had a benefit with antidepressant over placebo above 3 points, but only if the baseline HDRS score was above 28. Since most patients in most studies had lower scores, the authors concluded that antidepressants are not giving more benefit than nonspecific placebo effects in most patients.
The statistical floor effect is this: If I start with a HDRS score of 50, and improve by 50% to a final score of 25, that is a 25 point improvement. But if I start with a HDRS score of 20 and improve by 50% to a final score of 10, that is only a 10 point improvement. The same relative effect of 50% improvement produces a smaller absolute effect when the depression scale starts at a lower number. There is a floor to how much benefit one can see in absolute depression scores. It may be, then, that the apparent lower benefit from mild depression has a similar explanation. Recently, my colleague Paul Vohringer and I conducted an analysis of this question; we converted all the studies in the above meta-analysis to a percent improvement score on the HDRS, rather than absolute change in HDRS points. In so doing, we also converted the 3 point difference – the standard of clinical significance – to a relative equivalent, which turned out to be about 12% difference between antidepressant and placebo.
In an analysis which is in press, we found the following: the difference between antidepressant and placebo for mild depression [HDRS below 24] was only about 5%, which is far below the clinical significance threshold. For severe depression [HDRS above 28] it was 16%, which is above that threshold. So far, our results agree with the original meta-analysis. But for moderate depression [HDRS between 24 and 28], the result was a 12% difference. Thus, when corrected for relative change, antidepressants have clinically significant benefits for moderate depression as well as severe depression, and this would include the majority of patients included in the FDA studies. The original meta-analysis concluded, with much ballyhoo in the mainstream media, that antidepressants are not effective in most patients, except in a minority with the most extreme depressions. That conclusion did not correct for the clear statistical fact that it is more difficult to show absolute differences with lower depression scores – a floor effect. After correcting for that floor effect by looking at change in relative, not absolute terms, these results would indicate that antidepressants are effective in many patients – at least acutely – except in the mildest depressions.
Differential rate of drug-attributable response according to initial severity of depression is an old idea. This effect was identified for the original tricyclic antidepressant drug imipramine (Tofranil). As shown in the chart, for almost 1000 patients those with the mildest severity actually did better with placebo than with drug. With increasing severity, the response rate declined with placebo and increased with drug. In the extremely severe cases, the rate of response to drug declined somewhat – these are the cases who more often need ECT. The Number Needed to Treat was excellent, between 2 and 3, in moderate to severe depression but was poor at 8-9 in the milder cases. For comparison, NNT is 8-11 for SSRI drugs in contemporary trials. The data shown in the chart come from Angst J, Human Psychopharmacology – Clinical and Experimental 1993; 8: 401-407. [NNT is a so-called clinimetric measure that tells us how many patients would need to be given the active drug in order to obtain 1 response that would not have occurred anyway with placebo. With NNT low is good.]
These data suggest that today’s most widely used antidepressant drugs are less efficacious than the early drugs. They were marketed not on efficacy but on tolerability and safety. Efficacy for the SSRI drugs always was unimpressive. Ironically, the wide use of such drugs now needs to be considered as a cause of the new epidemic of so-called treatment resistant depression – you know, the cases for whom Abilify and Seroquel are being touted. That is a true iatrogenic train wreck – prescribing antipsychotic drugs for nonpsychotic depressed patients because they did not respond to second rate antidepressants!
I recently saw a guy in our little clinic – a 50+ year old alcoholic who had been living in a half-way house for six months. He was no derelict, a construction worker whose wife had “taken it all” in the divorce so he decided to check in and sober up to stop “wrecking trains.” The point is, he was 4+ depressed [visible in the waiting room], and it wasn’t grief [“glad to be free of her”]. I tried the Celexa trip, going up on the dose when he didn’t respond. Still nada. So I treated him with Elavil in a full dose as if it were 1974 when I showed up for my residency. At one month, he walked in and said, “I think that was it.” He was glad. I was too, but I think I was more mad. I was so peripheral to the world of that kind of case after I left Emory that most of what I knew was “book learning.” The slide you’re showing and your comments are not widely known. I didn’t know them until I started looking. General Psychiatrists and PCPs don’t know that either.
I knew that guy was different when I went to the waiting room the first time – different from the garden variety “I’m depressed” cases that haunt clinics like the one I work in. He was the one that I worried about and would’ve called if he missed an appointment. So it’s not just the fairy tale about SSRIs, it’s the DSM-III version of “MDD” that’s awry. He’s lumped in there with everyone else. Even an old psychoanalyst like me who takes a too long history of everyone didn’t put any weight on his marital loss, losing everything he owned, or even his substance abuse – forget the psycho-social, precipitants or not, this was a bio case from the waiting room on.
Another kind of cute thing. After he improved, he called me aside and fumbled around but was worried about his “nature.” I was afraid to change the medication given the dark hole he’d just come out of and said “let’s see.” The next time I saw him, he had continued the medication, but announced with a smile that he guessed he’d “just gotten out of practice” – “nature” was back in business.
My point.
Needs to be in the AJP!