The Texas Medication Algorithm Project:
Development and Implementation of the Schizophrenia Algorithm
[full text on-line]
by John A. Chiles, M.D., Alexander L. Miller, M.D., M. Lynn Crismon, Pharm.D., A. John Rush, M.D., Amy S. Krasnoff, M.A. and Steven S. Shon, M.D.
Psychiatric Services 1999 50:69-74.The Texas Medication Algorithm Project is a program designed to improve the quality of care of persons with serious mental disorders across sites in the Texas public mental health system and to create a uniform clinical environment from which cost estimates can be made. This paper describes the process of developing a pharmacological treatment algorithm for schizophrenia that addresses use of antipsychotics as well as other medications for side effects and co-existing symptoms. Input from clinicians, consultants, and consumers informed development of the algorithm, which was based on existing expert consensus guidelines. Information about the project can be found on the Internet at
www.mhmr.state.tx.us/meds/tmap.htm. The authors present and describe the original and current versions of the algorithm, outline the feedback process by which it will be refined, and discuss how new medications will be incorporated as they enter the market.
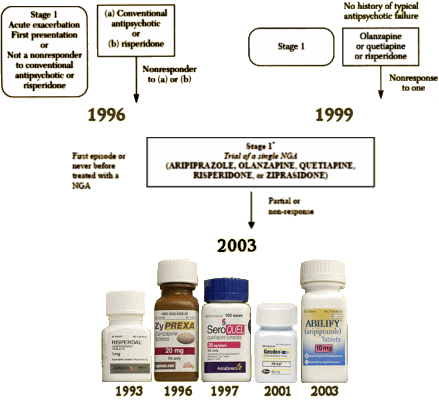
 The volumes of treachery and intrigue in the psychopharmaceutical industry of our time might rival the shelf space of Zane Grey’s stories of the old west, and the Texas Medication Algorithm Project tale stands above all the others – a truly Texas-sized story. Risperdal [Risperodone] is an antipsychotic drug introduced as a step forward in the treatment of Schizophrenia – a limited market of patients primarily treated in the public sector. When introduced in 1993, there were hopes that it would be more efficacious and less toxic than the first generation antipsychotics of the previous forty years. TMAP was a joint venture among academia, industry, and the State of Texas to select by expert opinion the medications used in its vast public system [prisons, clinics, Medicaid, etc.]. Starting with Consensus Guidelines prepared from afar, the TMAP group arrived at its own algorithms, then repeatedly revised them as new drugs became available [above]. TMAP went on the road, sending emissaries to other States and abroad – ultimately engaging multiple other States to set up similar programs. An enthusiastic Texas Governor Bush took the program to Washington when he was elected President in 2000.
The volumes of treachery and intrigue in the psychopharmaceutical industry of our time might rival the shelf space of Zane Grey’s stories of the old west, and the Texas Medication Algorithm Project tale stands above all the others – a truly Texas-sized story. Risperdal [Risperodone] is an antipsychotic drug introduced as a step forward in the treatment of Schizophrenia – a limited market of patients primarily treated in the public sector. When introduced in 1993, there were hopes that it would be more efficacious and less toxic than the first generation antipsychotics of the previous forty years. TMAP was a joint venture among academia, industry, and the State of Texas to select by expert opinion the medications used in its vast public system [prisons, clinics, Medicaid, etc.]. Starting with Consensus Guidelines prepared from afar, the TMAP group arrived at its own algorithms, then repeatedly revised them as new drugs became available [above]. TMAP went on the road, sending emissaries to other States and abroad – ultimately engaging multiple other States to set up similar programs. An enthusiastic Texas Governor Bush took the program to Washington when he was elected President in 2000.
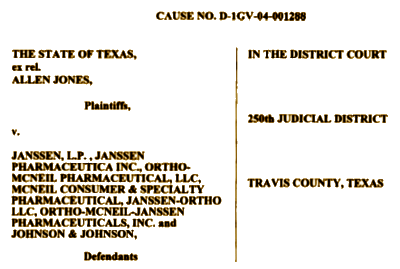
When Allen Jones in the Pennsylvania Office of the Inspector General found improprieties in travel vouchers connected to Pennsylvania’s dealings with TMAP and began to look around, his superiors tried to wave him off the case. When he persisted, he found himself on the sidewalk with the contents of his desk in a cardboard box, out of a job. On the long road to Austin, he won his suit against his former employers and the offending official ended up a convicted felon. Tracing the improprieties back to Texas and TMAP, he filed a whistle-blower suit against Johnson & Johnson in 2004 in Texas, later joined by the State of Texas in 2006. From what is available publicly, it appears that J&J had its fingers in and financed every piece of this story – those Guidelines from afar, the Texas Medication Algorithm Project experts, the wandering Texas emissaries, the traveling Pennsylvanians, etc. What makes this story stand out from all the other pharmaceutical misadventures is that it involved charity patients treated in a public system paid for by Medicaid funds using the most expensive possible medications with no scientific verification that they were more effective [and came close to bankrupting the taxpayer financed Texas Medicaid system].
When a Congressional investigation revealed in June that Dr. Joseph Biederman, a world-renowned child psychiatrist, had earned far more money from drug makers than he had reported to his university, he said that his interests were “solely in the advancement of medical treatment through rigorous and objective study.” Court documents reveal that Dr. Joseph Biederman, a renowned child psychiatrist, pushed Johnson & Johnson to fund a research center whose goal was “to move forward the commercial goals of J&J.”
But e-mail messages and internal documents from Johnson & Johnson made public in a court filing reveal that Dr. Biederman pushed the company to finance a research center at Massachusetts General Hospital, in Boston, with a goal to “move forward the commercial goals of J.& J.” The documents also show that the company prepared a draft summary of a study that Dr. Biederman of Harvard, was said to have written.
Dr. Biederman’s work helped to fuel a fortyfold increase from 1994 to 2003 in the diagnosis of pediatric bipolar disorder and a rapid rise in the use of powerful, risky and expensive antipsychotic medicines in children. Although many of his studies are small and often financed by drug makers, Dr. Biederman has had a vast influence on the field largely because of his position at one of the most prestigious medical institutions…
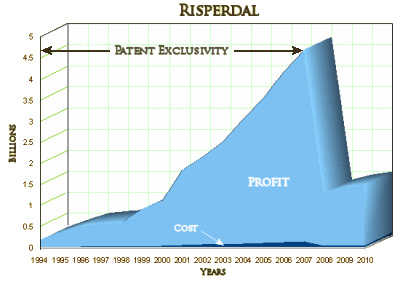 Through the Texas Medication Algorithm Project, Johnson & Johnson raided Medicaid and other public funds. Johnson & Johnson’s deal with Biederman involved giving children potentially toxic drugs to expand its customer base. Sometimes, it’s easy to get caught up in the intricacies of a story, and miss the essence of what happened. Johnson & Johnson lost all sight of the business they’re allowed to be involved in – stealing from the public coffers and over-medicating children to inflate corporate profits.
Through the Texas Medication Algorithm Project, Johnson & Johnson raided Medicaid and other public funds. Johnson & Johnson’s deal with Biederman involved giving children potentially toxic drugs to expand its customer base. Sometimes, it’s easy to get caught up in the intricacies of a story, and miss the essence of what happened. Johnson & Johnson lost all sight of the business they’re allowed to be involved in – stealing from the public coffers and over-medicating children to inflate corporate profits.


You wrote:
“… algorithms are ways to solve problems. This wasn’t a solution.”
You echo the feelings of many of us in reference to pharmacological psychiatry: We were taught that “medicine” promotes healing. Psychiatric drugs do nothing of the sort…
And so, a growing number of us are left shaking our heads, dumbfounded… while those who continue to drink the Kool-Aid call us “scientologists” or worse.
Duane
Why is there no mention of the elderly in nursing homes being harmed and killed with the same drugs. I provided Allen Jones with documentation which clearly proved that TMAPS was being utilized by Texas DHS. The state victimized me for daring to notice and complain. The nursing home chain, Mariner (also known as Living Centers of Texas) owned the pharma service provider company contracted with the state to self police, and the state did everything to help protect the nursing homes and the pharmaceutical companies. Disgraces in care and protection!
Brenda, that’s an excellent point! I did pull out children with Dr. Biederman’s contribution, but I failed to fractionate the TMAP impact which involved children, adults [even those with non-psychotic illnesses], and the elderly – a large target population for the Risperdal [and other Atypicals]. The drugs now carry a warning of dire side effects including death in the elderly. That’s another exaple of drug sales trumping patient safety and care. Thanks for the reminder…