If you have any more energy for Latuda®, there’s another study[ies] coming soon to a journal near you. A year ago, Sunovion announced another six week schizophrenia trial [80mg, 160mg] against comparator Quetiapine [Seroquel®] 600mg XR. But that was just a prequel to a one year study to look at relapse rates. In October, Sunovion published a press release with those results:
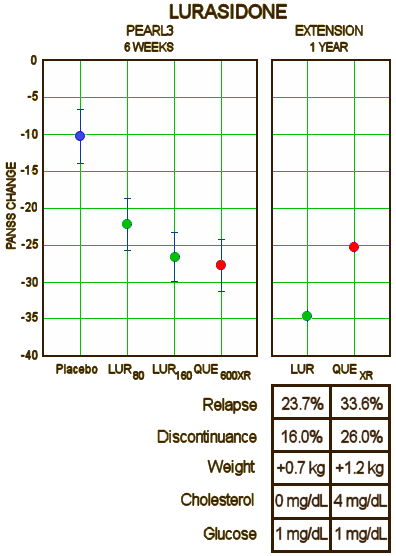

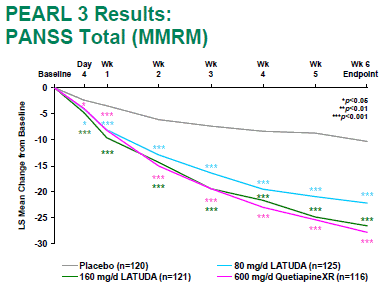
[Lest you’re curious who Dr. Steven G. Potkin works for, check this out]. While the results themselves are not yet published, they look pretty good in the press release. Latuda® held its own against Seroquel XR® in the 6 week trial, and outdid Seroquel XR® in the one year extended study. Obviously, the press release version is the best this study will ever look, but still, it looked pretty good. Here’s their slide from the 6 week prequel:
All the business wires carried it as "LATUDA® Shown to be Non-Inferior to SEROQUEL XR® in Risk for Relapse in a 12-Month, Double-Blind Extension." This study really is also an experimercial – competition with Seroquel XR® [still under patent] in the marketplace. I’m assuming that Quintiles is still involved in sailing this ship [Kalali is mentioned with this multinational study]. They’re steering Sunovion’s Latuda® in well travelled waters. First go head to head with the competition, accenting features that are advantages [low metabolic effects, no weight gain]. Then they begin the indication creep into the Bipolar/Depression market, hoping for off-label use for anxiety etc. It’s an old story by now, trail-blazed by Risperdal®, Zyprexa®, Seroquel®, Geodon®, Abilify®, and an army of lawyers etc.
| Clinical Trials in the works – Lurasidone
|
|
| Lithium Drug-Drug Interaction With Lurasidone | August 2008 |
| Lurasidone – A 6-week Study of Patients With Bipolar I Depression (Monotherapy) | April 2009 |
| Lurasidone – A 6-week Study of Patients With Bipolar I Depression (Add-on) | April 2009 |
| Lurasidone – A 24-week Extension Study of Patients With Bipolar I Depression | April 2009 |
| Clinical and Biomarker Assessment of Efficacy of Cognitive Remediation in Patients With Schizophrenia Stabilized on Lurasidone |
July 2010 |
| Lurasidone HCI – A 6-week Phase 3 Study of Patients With Bipolar I Depression | November 2010 |
| A Study of Subjects Switched to Lurasidone for the Treatment of Schizophrenia or Schizoaffective Disorder |
August 2010 |
| Major Depressive Disorder (MDD) With Mixed Features – Flexible Dose | September 2011 |
| PEARL Schizophrenia Maintenance | September 2011 |
| Bipolar Maintenance Study of Lurasidone Adjunctive to Lithium or Divalproex | June 2011 |
| Major Depressive Disorder With Mixed Features – Extension | September 2011 |
| Major Depressive Disorder With Mixed Features | January 2012 |
I doubt they’re putting this much effort into a "me too" drug for schizophrenia. Hardly enough patients. It’s much more likely that they’re aiming to tag along with the Atypical Antipsychotics’ success as non-specific "nerve pills" being prescribed for insomnia, anxiety, depression, etc. by doctors of all ilks. I found myself thinking that this particular study specifically targeted the still patent-protected Seroquel XR® [now being heavily advertised as a depression add-on] in hopes of being branded "the newer, better Seroquel." But even at that, it’s unclear to me how Sunovion/Quintiles/whomever plans to make this into a blockbuster drug at $14.00/pill given that there are ads like this one all over the Internet.

I guess that’s unclear to everyone, but that’s not my reason for sticking with this Latuda® story as it unfolds. This is the first Atypical Antipsychotic that’s come out since I’ve been following this aspect of psychiatry, and I’m just curious how it all works, or if it can possibly keep going like it has for the last twenty-five years with recurrent excitement over the latest drug to hit the market followed by a dose of disillusioning reality. And something else – when I googled "latuda quetiapine" to find the reports on this study, most of the hits were investment sites and stock analysts [one of which finally lead me to the press release itself], not medical sites.
One wonders how long this kind of thing can continue: academic professors making drug commercials; speaker’s bureaus dispatching psychiatrists to fly around the country hawking drugs to primary care physicians; overvaluation of minor receptor affinity differences discussed as if they really do directly correlate with efficacy and side effects; Clinical Research Organizations coordinating large drug trials spread around the world in record time; a mindless NIMH Director cheer-leading each new fad with naive enthusiasm; an ideologically mired DSM-5 Task force revising a supposedly non-ideological diagnostic system; professional organizations and journal editors with blind eyes to scientific improprieties and outright corruption; drugs like Viibryd® and Latuda® trying to follow road-weary pathways; and a profession at large in denial that the days of wine and roses are over and a time for re-evaluation is at hand.
Your penultimate paragraph, in my opinion, says everything one needs to know about modern psychiatry.
Unfortunately, it’s an uphill battle to get the word out to the rest of our colleagues.
Not certain what penultimate means, but clearly understand your posts and follow them qd. I typically repost your Blog on my Twitter Account, in an effort to “spread the word” Complete agreement with above comment by SteveBMD – David Bransford MD
had to look up penultimate to figure out that I agree with Steve and was going to make the same point but wouldn’t have been able to come up with that word! Penultimate is much fancier than “your next to last paragraph” but I share the sentiment.