I’m in something of a cul-de-sac and I don’t see any way out other than to explore the territory. At least I know how I got here. It was those Call-Notes from the Janssen Trial in Texas. I went there understanding how I felt about what Janssen had done, but I wanted to hear about it first hand. Well, that sure happened, but I came home with a new disquieting feeling. It was all those Call Notes, the notes the Janssen Sales Reps wrote after each call they made to the doctors on their "target lists." In the Trial itself, they were presented as evidence to prove that Janssen was promoting Risperdal off label to Child Psychiatrists [among other things]. They not only had no indication for children and adolescents, but they were promoting it for things that they had no approved indication for even in adults. There was one training slide, a sales aid that they showed us repeatedly [for obvious reasons]:
-
Child and Adolescent Psychiatrists
-
Provide with treatment under 13
-
Most diagnosed with behavior disorders or mood disorders
-
No indications!!!
-
Sell on symptoms not diagnosis
So they knew exactly what they were doing. Along those lines, there was one particular Call Note that stood out from the others:
"Continued with John’s call and spoke of new areas to use Risperdal. Used JAACAP to show augmentation to stimulants with low dose – low dose Risperdal for hostility/aggression. This seemed to spark some interest, so we might need to elaborate here since he sees so many kids."
I searched PubMed for the Journal of the American Academy of Child and Adolescent Psychiatry AND Risperidone, and there was only one article that fit the bill:
a placebo-controlled pilot study.
by Armenteros JL, Lewis JE, and Davalos M
Journal of the American Academy of Child and Adolescent Psychiatry. 2007 46(5):558-65.
ClinicalTrials.gov Identifier: NCT00297739
OBJECTIVE: To evaluate the effects of risperidone augmentation for treatment-resistant aggression in children with attention-deficit/hyperactivity disorder [ADHD].
METHOD: Twenty-five children [ages 7-12 years] with attention-deficit/hyperactivity disorder [ADHD] and significant aggressive behaviors were randomized to risperidone or placebo for 4 weeks for this double-blind study. Subjects were already in treatment with a constant dose of psychostimulant medication. The primary efficacy measure was change from baseline in the Children’s Aggression Scale-Parent [CAS-P] and -Teacher [CAS-T] total scores.
RESULTS: The mean risperidone dose at endpoint was 1.08 mg/day. For the CAS-P total score, a significant difference was found [chi(1)(2) = 4.30, p < .05] with 100% of risperidone subjects improving by more than 30% from baseline to endpoint, whereas only 77% of the placebo group reported a similar response. No differences were found on the CAS-T total score. For the CAS-P and CAS-T, no significant interaction was found between treatment group and time. Rates of adverse events did not differ significantly between groups.
CONCLUSIONS: Risperidone treatment appears to be well tolerated and modestly effective when used in combination with psychostimulants for treatment-resistant aggression in children with ADHD.
I had to look, and there it was:
Accepted November 29, 2006.
Dr. Armenteros is in private practice, Coral Gables, FL; and Dr. Lewis and
Ms. Davalos are with the University of Miami Miller School of Medicine.
This study was supported by Janssen, LP…
Dr. Armenteros is in private practice, Coral Gables, FL; and Dr. Lewis and
Ms. Davalos are with the University of Miami Miller School of Medicine.
This study was supported by Janssen, LP…
And the data?
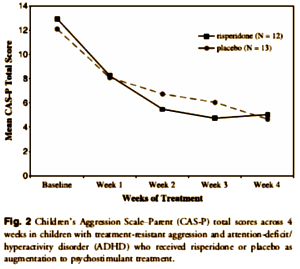
I guess we’re wondering what it was about this article that "spark[ed] some interest"? I, for one, didn’t feel sparked by this industry-funded, underpowered, essentially negative study done by a private practitioner handed out by a drug rep to support the off label use for a medication for behavior control. What I was sparked to wonder is how it made it into the Journal of the American Academy of Child and Adolescent Psychiatry [JAACAP] in the first place.
But then I remembered something! I’m on the wrong track. This article probably wasn’t what "JAACAP" referred to. The date is all wrong. This is a Call Note from Tiffany Moake who was a Sales Rep in 2003-2004. She most have been referring to data from the presentation Dr. Joseph Biederman signed onto at the American Academy of Child and Adolescent Psychiatry in 2002. And when I say signed on to, I mean that literally [bipolar kids: postscript, detestable?…]. And it’s part of a bigger story too. Janssen had it’s eyes on people using Risperdal for behavior control in disruptive children from the start. Starting in 1997, a Janssen group, Risperidone Disruptive Behavior Study Group, did a large study using Risperdal for behavior control in retarded children [Aman et al, finally published in 2002]. The FDA wouldn’t approve that usage. Amazingly, that data was reused by Dr. Joseph Biederman when he got started on his Bipolar Child epidemic as evidence for using Risperdal in his patients. It’s a sleazy side story covered in these earlier posts well worth reviewing if you don’t know the story.
- bipolar kids: an all too familiar lingo…
- bipolar kids: biedermania and super angry/grouchy/cranky irritability…
- bipolar kids: postscript, detestable?…
So this Call Note is from a time when they were using Joseph Biederman’s [guest author!] presentation at the meeting to detail Child Psychiatrists, a time before Biederman’s Bipolar Child story grabbed hold and legitimized [in the minds of many] treating disruptive kids with Risperdal. It wasn’t the lousy study in PubMed that was being detailed after all, it was the ghost-written study that Dr. Biederman signed onto right after J&J funded his Center at Mass General [it’s amazing to me how these stories keep running into each other].
Janssen was standing on it’s head to find a way to push Risperdal for controlling hostility and aggression in children – retarded kids, ADHD kids, autistic kids, "Bipolar" kids, whatever it took. It was a huge market and they went for it full bore – approvals or not. My point is that the doctor in question apparently went for the idea ["spark"] too. First, Janssen was hell bent on finding a way to medicate disruptive kids with antipsychotics, but it obviously fell on receptive ears and has morphed over the years into a problem of its own – mainly discussed these days as the massive overmedication of children in Foster Care and children with disabilities.
"Had an entire waiting room of foster kids; Rosemary said she sees at least 10 Risperdal prescriptions go out a day."
That Janssen and PHARMA in general have gone in every direction possible to legitimize and popularize using antipsychotics with hostile, aggressive, or disruptive children seems without question, to their shame. But I knew that already. What lingered with me is that the Call Notes were about sales calls to doctors who were receptive to what they were saying – to using Risperdal as a chemical straight-jacket for difficult kids. Even worse, using Risperdal routinely with disabled and disenfranchised children. There’s something very wrong about all of this, even beyond the obvious exposure to the toxic adverse effects. Growing up drugged can’t be a good idea. It sounds like we’re trying to make up for not providing these children with adequate services and care by prescribing medications to keep them quiet. Janssen may be opportunizing on this state of affairs and physicians may be overprescribing because they don’t know what else to do with a waiting room full of such children. The rest of us may be decrying overmedication of kids. But the bottom line may well be a system overwhelmed without adequate resources. What a mess…
Mickey, you know once I write enough of these names, they stick in my mind. One in your post rang bell (as many do, birds of a feather after all)
Conflict of interest!: FDA Psychopharmacologic Drugs Advisory Committee: AstraZeneca & child psychiatrist, Chair Armenteros
http://bipolarsoupkitchen-stephany.blogspot.com/2009/04/conflict-of-interest-fda.html
AstraZeneca P.L.C. paid Florida child psychiatrist Jorge Armenteros to talk to other doctors about prescribing Seroquel, the company’s powerful antipsychotic.
And until yesterday, Armenteros also was listed as the chair and a voting member of a Food and Drug Administration advisory committee with a lot of power over Seroquel, which generated $4.45 billion in sales last year for AstraZeneca, whose U.S. headquarters are in Wilmington.
http://bipolarsoupkitchen-stephany.blogspot.com/2009/04/usa-fda-cannot-be-trusted-drug-approval.html
The call notes will probably haunt you for a long time. Because it’s like waching a crime unfold and the victims were so innocent.
I felt serious betrayal and anger when I read the internal Zyprexa documents which included notes like that. These notes portray reality with pharma reps and doctors offices, and frankly ones who sell drugs this way to earn a living and get bonuses,and territories….well let’s just say crimes like the ones we read in these notes sound purposeful, deliberate and with intent.
So they are guilty, guilty of a crime committed against children in the name of selling a pharma product.
As for the FDA, and how these are all tied together…. leaves me saying what many people say (and doctors I hope):
Who do you trust?
Too bad the inner-circle keeps validating these types by being members of the APA, believing in the DSM5 or allowing drug pharma reps tell them about meds…until the inner circle cleans itself up and says NO MORE the ones like Nemeroff et al and the ones listed in your post will just keep on keeping on just like the Big Pharma cos….business as usual and the patients are just part of the road ran over by their steamrollers.
*I should comment the reason for my anger at the Zyprexa internal notes (another antipsychotic illegally marketed to children) is because just like Risperdal, that was off-label rx’d to my then 11 year old, in fact the doctor removed the Risperdal that caused massive reaction and replaced it with Zyprexa! she then fell into their Viva Zyprexa sales pitch being she was to take it at 5pm…
As Robert Frost is quoted as saying about life–“it goes on”.
Who do you trust?
http://youtu.be/ZItVOxkXinw
The pharmaceutical industry & mainstream psychiatry have created a symbiotic nightmare relationship the public and consumers never get to wake up from.
Just think for a moment what a 100 bn dollars a year (low ball estimate) could provide an overwhelmed system without adequate resources.
Of course the game is rigged from top to bottom, with no safety net or accountability built into it’s structure : and whatever cosmetic changes the voices of the brave few can muster appear to be doing little to slow down or derail a runaway corporate freight train monopoly of power, corruption, and unquenchable greed…
Reality is often times a very bitter & hard pill to swallow…no matter how it’s marketed or packaged…
LOOK at this:
http://mghcme.org/courses/course-detail/child_adolescent_psychopharmacology_2012
Click faculty
Click CME Information
Biederman, Wilens, Wozniak, Spencer, Wagner (Wagner from PAXIL 329 ) are all part of the faculty teaching CME
Child & Adolescent Psychopharmacology 2012Annah Abrams, MDPradeep G. Bhide, PhDJoseph Biederman, MDBarbara Coffey, MD, MSEsther J. Dechant, MDAlysa E. Doyle, PhDOliver Freudenreich, MDDaniel A. Geller, MDPaul Hammerness, MDAude Henin, PhDDina R. Hirshfeld-Becker , PhDMichael S. Jellinek, MDGagan Joshi, MDMichael Monuteaux, ScDRoy H. Perlis, MDJefferson B. Prince, PhDRonald Schouten, MD, JDThomas J. Spencer, MDCraig Surman, MDKaren Wagner, MD, PhDTimothy E. Wilens, MDJanet Wozniak, MD
Now how can this be? Biederman and pals were sanctioned by Harvard for the Grassley investigation of non disclosure of millions of pharma income; how can these people get away with teaching doctors pharmacology for kids??!!
Please write about this! thanks