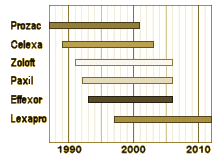
 While historians have the advantage of freedom from contemporary drama and knowing the outcome, they lose the passion of the moment. The moments after the introduction of the DSM-III were certainly dramatic at the time. For all intents and purposes, with the DSM-III, psychiatry had declared itself a biological science, free from the shackles of conjecture and speculation, but it was the introduction of Eli Lilly’s Prozac in 1987 that instantiated this change in the general culture – something of a wonder drug. – the debut of the age of a new psychopharmacology. GSK’s Paxil was something of a late comer.
While historians have the advantage of freedom from contemporary drama and knowing the outcome, they lose the passion of the moment. The moments after the introduction of the DSM-III were certainly dramatic at the time. For all intents and purposes, with the DSM-III, psychiatry had declared itself a biological science, free from the shackles of conjecture and speculation, but it was the introduction of Eli Lilly’s Prozac in 1987 that instantiated this change in the general culture – something of a wonder drug. – the debut of the age of a new psychopharmacology. GSK’s Paxil was something of a late comer.
According to the documents, GSK began to push sales of Paxil in the early 1990s with an extensive ghostwriting program run by the marketing firm Scientific Therapeutics Information (STI). For instance, STI wrote a proposal to organize GlaxoSmithKline’s Paxil Advisory Board Meeting in 1993 at the Ritz Carlton Hotel in Palm Beach, Florida. STI chose Dr. Charles Nemeroff of Emory University as their speaker to lay out the meeting’s agenda and objectives. Dr. Nemeroff apparently led discussions on how to “evaluate clinical research/promotional programs” and “generate information for use in promotion/education.” [Attachment A]


Another sign of those times, alliances between academia, the NIMH, and industry were seen as valuable, encouraged. Translational science was big, and the NIMH has a number of programs designed to foster university/private sector collaboration. Dr. Insel came to the NIMH in 2002 after directing such a program in Atlanta at Emory. In 2003, Dr. Nemeroff received a collaborative grant from NIMH, the EMORY-GSK-NIMH COLLABORATIVE MOOD DISORDERS INITIATIVE.
This application, in response to RFA: MH-03-008 (National Cooperative Drug Discovery Groups for the Treatment of Mood Disorders or Nicotine Addiction, NC 336-MD/NA), proposes the creation of"The Emory-GSK-NIMH Collaborative Mood Disorders Initiative." This unique opportunity to accererate antidepressant drug development brings together expertise of three complementary research groups: the Emory University School of Medicine Department of Psychiatry and Behavioral Sciences, the Mood and Anxiety Disorders Program at NIMH and the Center for Excellence in Drug Discovery (CEDD) in Psychiatry of GlaxoSmithKline (GSK), one of the largest multinational pharmaceutical companies. The two major goals of the current application are the development of innovative new models for basic and clinical research in mood disorders and the intensive scrutiny of 5 novel GSK antidepressant candidates in preclinical and clinical paradigms…
… In 2004, Emory investigated Dr. Nemeroff’s outside consulting arrangements. In a 14-page report, Emory’s conflict of interest committee detailed multiple “serious” and “significant” violations of university procedures intended to protect patients. But the university apparently took little action against Dr. Nemeroff and made no effort to independently audit his consulting income, documents show. Universities, too, can benefit from the fame and money the deals can bring — a point Dr. Nemeroff made in a May 2000 letter stamped “confidential” that he sent to the dean of Emory’s medical school. The letter, which was part of a record from a Congressional hearing, addressed Dr. Nemeroff’s membership on a dozen corporate advisory boards [some of the companies’ names have since changed].“Surely you remember that Smith-Kline Beecham Pharmaceuticals donated an endowed chair to the department and that there is some reasonable likelihood that Janssen Pharmaceuticals will do so as well,” he wrote. “In addition, Wyeth-Ayerst Pharmaceuticals has funded a Research Career Development Award program in the department, and I have asked both AstraZeneca Pharmaceuticals and Bristol-Meyers [sic] Squibb to do the same. Part of the rationale for their funding our faculty in such a manner would be my service on these boards”…
For all his fame in the world of psychiatry, Dr. Nemeroff has faced ethics troubles before. In 2006, he blamed a clerical mix-up for his failing to disclose that he and his co-authors had financial ties to Cyberonics, the maker of a controversial device that they reviewed favorably in a journal he edited. The Cyberonics paper led to a bitter e-mail exchange between Dr. Nemeroff and Claudia R. Adkison, an associate dean at Emory, according to Congressional records. Dr. Adkison noted that Cyberonics had not only paid Dr. Nemeroff and his co-authors but had also given an unrestricted educational grant to Dr. Nemeroff’s department. “I can’t believe that anyone in the public or in academia would believe anything except that this paper was a piece of paid marketing,” Dr. Adkison wrote on July 20, 2006.
Two years earlier, unknown to the public, Emory’s conflict of interest committee discovered that Dr. Nemeroff had made more serious blunders, including failing to disclose conflicts of interest in trials of drugs from Merck, Eli Lilly and Johnson & Johnson. His continuing oversight of a federally financed trial using GlaxoSmithKline medicines led Dr. Adkison to write Dr. Nemeroff on July 15, 2004, that “you must clearly certify on your annual disclosure form that you do not receive more than $10,000 from GSK.” In a reply dated Aug. 4, Dr. Nemeroff wrote that he had already done so but promised again that “my consulting fees from GSK will be less than $10,000 per year throughout the period of this N.I.H. grant.”
When he sent that letter, Dr. Nemeroff had already earned more than $98,000 that year from GlaxoSmithKline. Three weeks later, he received another $3,844.56 for giving a marketing talk at the Passion Fish Restaurant in Woodbury, N.Y. From 2000 through 2006, Dr. Nemeroff earned more than $960,000 from GlaxoSmithKline but listed earnings of less than $35,000 for the period on his university disclosure forms, according to Congressional documents…
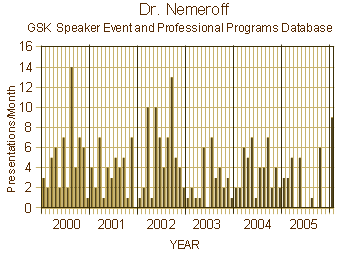
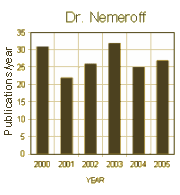 So in 2000, Dr. Nemeroff’s extensive ties with Industry were noted at Emory. In 2004, Emory’s Conflict of Interest Committee had investigated him, found a number of improprieties, and insisted he sign a pledge that he would not make > $10,000/year from GSK specifically – a pledge he ignored. It was not until 2006 when he omitted Conflict of Interest disclosure in an article in a journal he himself edited and he was "busted" and removed as editor that Emory acted, but he remained chairman until 2008 when Senator Grassley investigated and showed the actual extent of his GSK involvement. Meanwhile, his publication rate during this period of time was uneffected. Meanwhile, he was on the Boards of many other companies and an active member in a lot of other psychiatric organizations.
So in 2000, Dr. Nemeroff’s extensive ties with Industry were noted at Emory. In 2004, Emory’s Conflict of Interest Committee had investigated him, found a number of improprieties, and insisted he sign a pledge that he would not make > $10,000/year from GSK specifically – a pledge he ignored. It was not until 2006 when he omitted Conflict of Interest disclosure in an article in a journal he himself edited and he was "busted" and removed as editor that Emory acted, but he remained chairman until 2008 when Senator Grassley investigated and showed the actual extent of his GSK involvement. Meanwhile, his publication rate during this period of time was uneffected. Meanwhile, he was on the Boards of many other companies and an active member in a lot of other psychiatric organizations. Reviewing all of this, it’s hard to imagine that it all really happened, and that it took a Senate Investigation to finally put an stop to it, or at least slow it down. It was quite a juggling act, and it went on for years. I doubt there’s another story like this in all of medicine. One could legitimately ask what was the point? Was this about money? or power? or prestige? Whatever it was about, it’s part of the back-story of psychiatry that continued for a quarter of a century and plagues us yet today.
FYI http://www.medscape.com/viewarticle/764740_print
From Medscape Psychiatry
DSM-5: Finding a Middle Ground
Nassir Ghaemi, MD
“So, the reliability of DSM-5 criteria seems to have declined compared to DSM-III. Is this a problem? It might be, but it might not be.”
“Hyman noted that DSM-III actually hinders science. Researchers have difficulty getting funding from the National Institutes of Health or publishing papers that go outside DSM criteria: “For example, it was very hard to get a grant to test the hypothesis that maybe the apparent comorbidity of multiple anxiety disorder and mood disorders was just that there was a single underlying process or single disorder that got expressed with different symptom complexes in different times in life.”
There was a name for that condition — neurotic depression — and Sir Martin Roth, the great British psychopathologist, warned repeatedly in the 1970s and 1980s that it would be a mistake for DSM-III to remove it. DSM-III made that mistake, and the field has since acted like it would be a sin to study the matter any further.
“I think we should have a new DSM just for research: a system of Research Diagnostic Criteria (RDC), like what was created in the 1970s that led to DSM-III to begin with. I’ve started that process with my colleagues in the world of bipolar disorder research. We will publish a new RDC for bipolar disorder within the coming year — before DSM-5, I hope. If we do so, I hope that colleagues in other specialties in psychiatry will produce similar RDCs.
With these new publications, psychiatry may then be in a position for real advance. We will then have 3 nosologies, all complementary to each other and able to improve the others:
DSM-5: a nosology based on a mix of research, economic concerns, social preferences, and professional consensus that is used for basic practice, insurance reimbursement, and short-term consensus.
RDOC: a nosology based solely on biological research that is used for research.
RDC: a nosology based solely on clinical research that is used for research.”
The NIMH grant is also an affront to taxpayers.
So far, only one professional organization has had the spine to sanction Dr. Nemeroff. In late 2009 the American College of Neuropsychopharmacology (ACNP) applied a 2-year sanction on Dr. Nemeroff. This action was taken in response to a formal complaint to the Ethics Committee of the College. The complaint was filed by Dr. Robert Rubin of UCLA and me. Nemeroff was banned from participation in College activities, committees, and conference presentations for 2 years. The NIH review committee that awarded the new grant contained a number of ACNP members. The administrative leader of NIMH, Thomas Insel, also is an ACNP member. It leaves us scratching our heads why they all would do that, especially as the science is poorly framed and poorly operationalized. See comments here for more on that.
Interesting. Commenter Ally apparently posted something too pointed about Nemeroff and the site admin hid it.
Someone was doing damage control on behalf of Nemeroff by complaining to the site administrator. I recall reading the comment when it was up there… it was disapproving of Nemeroff but it wasn’t over the top.
Oooo, mustn’t distress the silverbacks!