Study 329 has an extensive literature of its own familiar to most people with any interest in the relationship between the pharmaceutical industry and acadenic psychiatry. For the moment, I’d like to hold my focus on the raw data finally posed this month [the Appendices below]: The top four entries are summaries released after the successful New York suit in 2004, but the raw data was only recently released [August 2, 2012] after an inquiry by Peter Doshi [a movement…]. In an earlier post [the lesson of Study 329: the basics…], I extracted the raw data in Appendix C: and showed that there was no separation of the Placebo and Paroxetine groups [others have done the same using more sophisticated software and the LOCF correction method].
| Unipolar major depression: Study 329
|
|||
| table of contents
|
posted in
|
file
|
|
| Study synopsis acute | 2004 | PDF (0.03Mb) | |
| Study synopsis continuation | 2004 | PDF (0.03Mb) | |
| Full study report acute | 2004 | PDF (0.97Mb) | |
| Full Study report continuation | 2004 | PDF (0.56Mb) | |
| Appendix A: Protocol | 2012 | PDF (19Mb) | |
| Appendix B: Patient Data Listings by Demographic | 2012 | PDF (18Mb) | |
| Appendix C: Patient Data Listings by Efficacy | 2012 | PDF (18Mb) | |
| Appendix D: Patient Listings of Adverse Events | 2012 | PDF (8Mb) | |
| Appendix E: Patient Data Listings of Vital Signs | 2012 | PDF (3.5Mb) | |
| Appendix F: Patient Data Listings of Lab Values | 2012 | PDF (23.5Mb) | |
| Appendix G: CRF Tabulations by Patient | 2012 | PDF (53Mb) | |
| Appendix H: CRF for Adverse Experiences | 2012 | PDF (60Kb) | |
What does the raw data tell us about the adverse effects, specifically suicidality? This is what the article said – 11 serious adverse events. Appendix H: lists the case numbers of the Serious Adverse Events [the ones with red asterix were the 11 taking Paroxetine]:


Here are the comments on those cases from Appendix G: The lighter oneswere the ones I called, one more than they listed. They didn’t count 329.001.00065:
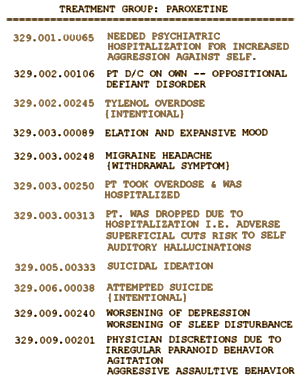
[Extracted from Appendix G:]
Then in Appendix D: they listed these Adverse Events under Emotional Lability:
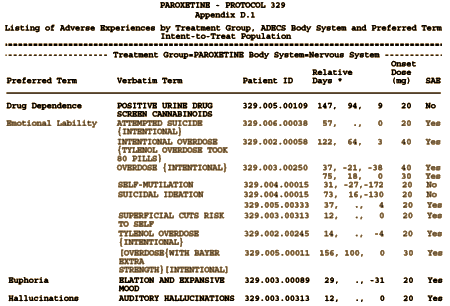
[truncated to fit the page. Click for the full table]
9 suicidal events in 8 patients. The difference seems to be in the Relative Days column. In the article, they only counted the information from the first eight weeks, even though the subjects continued on medication thereafter – adding 329.002.0058, 329.004.00015, and 329.005.00011. The fact that they cut-off the Adverse Events at 8 weeks is not mentioned in the article. Below is the comparison among Placebo, Imipramine, and Paroxetine from Appendix D: for Hostility and Emotional Lability [AKA Suicidality]:
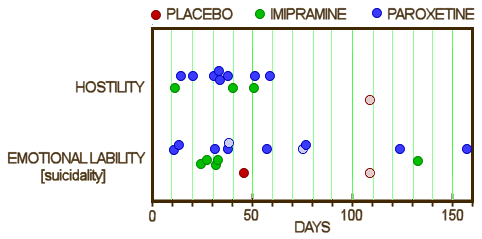
the lightened dots are two adverse events in the same subject
“let the science shine through”
I would make one further respectful, but strong, suggestion.
Look at the question that it appears that Dr. Keller, and possibly Drs. Ryan and
Wagner and Dulcan, appear seem most intent be dismissed. Not even be raised.
And it’s NOT, to my mind, the question of whether SSRIs can increase the risk for completed suicide in some individuals.
The question of whether or not SSRIs increase the risk of completed suicide is an important one. However, I would NOT focus on this question.
There is a fundamental question raised by the available documents surrounding Study 329. One that is important, and separate from the question above.
One that data transparency would greatly help, but in order to establish why you need data transparency you need to raise the issue that the process of how the safety data was handled was possibly poor enough to suggest the need.
That is the question I believe they wish to pretend does not exist or dismiss from the start under the umbrella of differences in scientific opinion. That is the forbidden ground.
Because what happens to AACAP’s journal and practice parameters and the authors of all those works if that gets called into question? We din’t have the kind of access there we do for Study 329.
Not evidenced based, but opinion: that is the finger in the dyke.
I think that Keller’s correspondence is suggestive of that point of view. And, looking at Keller’s career he appeared to have a keen understanding of how academic child psychiatry functions.
The question:
How the presentation of the safety information was addressed by Drs. Wagner, Ryan, Keller, Duncan, and by extension JAACAP/AACAP.
I believe this is worthy of your most concentrated focus.
Good scientists and clinicians can look at the raw data from 329, it’s 2 sister studies, and the multiple papers on the topic of SSRIs and completed suicide and come to different conclusions. Drs. Ryan and Wagner may be correct that the SSRIs do not increase this risk of completion, they may even be able to make a cogent argument that the totality of available data through 2012 supports their contention. Or they may be wrong.
Either way, that does not change the question of how that safety data was handled and presented in Study 329 and the 2001 JAACAP paper.
There is the expectation that primary authors on a paper, and the editor who publishes that paper, have the responsibility to be sufficiently aware of the raw data of the study, and then to represent that data in the results section, a way that would withstand scrutiny if the proprietary raw data and reviewers comments were hypothetically to be made public.
This is what Keller in his email in 2004 (about the safety data) to Ryan, Wagner, et appears most concerned about, and most adamant that there be no questioning of. He doesn’t focus on what the data actually shows, but rather that there must be 100% endorsement that the primary authors were fully aware of what they should have known and of how the paper handled the safety information. And, talks explicitly about the added importance of those who signed off on the response to Jureidini. He lays out very very what others could conclude if that were not 100% endorsed.
The data are highly suggestive that at the very least paroxetine could lead to psychiatric side effects that it would be worth monitoring for when putting an adolescent on paroxetine. Regardless of the risk of completed suicide. There is no replacement for the primary authors and editor being aware of the data in the study relevant to this and then presenting it in the results section in a way that bears scrutiny (it certainly did not beat the scrutiny of the JAMA and JACAP reviewers). The responsibility for the second is also shared by the senior editor.
It was the question of how those responsibilities were handled that appeared to be of great concern to Keller in his email to Ryan, Wagner, et al. in 2004.
I would strongly suggest that it be of great concern to us.
Then let others draw their own conclusions about implications, or not, about their handling of raw data and its presentation subsequent work where we have so little of the access to the data and the process that was afforded in Study 329 and related works.
JAACAP/AACAP appears to have never verbalized a question about the position that Keller wished 100% endorsed. It does not appear to have addresses the question about the handling of the safety data in Study 329, the 2001 paper, and the Jureidini response. The question of the handling, NOT of the conclusions drawn, which is worthy of collegial scientific discourse.
It’s striking to me to see the many euphemisms utilized for suicidality or suicidal ideation or dark thoughts or whatever you want to call it.
Anonymous, as a practical matter, clinicians, even psychiatrists, are terrible at monitoring anybody for anything on any psychiatric drugs. The idea that “monitoring” ameliorates a risk as serious as this is a pipe dream.
Surely the fact that paroxetine has ANY strongly adverse effects in adolescents indicates it should not be prescribed for them? (It has strongly adverse effects in adults, too, which also have been buried by dishonest literature and the admonition to “monitor.”)
AACAP appears to possibly have made statements ( through words, or actions, about Drs. Dulcan and Wagner):
“Mina K. Dulcan, M.D., editor extraordinaire, 1997-2007.”
“Mina K. Dulcan, M.D., editor extraordinaire, 1997-2007.”
“Her decisions and leadership were always thoughtful and measured. She chose her battles well, stood her ground, and let the science shine through.”
– Virginia Anthony
http://psycnet.apa.org/psycinfo/2007-18374-001
http://www.aacap.org/galleries/aacap_news/virginia_anthony_exec_director_of_aacap_retires_after_39_years.pdf
http://aacap.org/cs/life_members/an_open_letter_to_virginia_q_anthony
http://www.aacap.org/cs/root/developmentor/understanding_how_aacap_works
(note the makeup of the executive committee)
The suicidal behavior can be blamed on “depression”. I think there should also be a group who isn’t “depressed” that is given the medication to see what the medication does to people who aren’t disturbed. One of the most unscientific assumptions in biological psychiatry is that the brains of mentally disturbed persons are significantly different from the brains of unaffected people; therefore, ipso facto, if a person experiences a manic episode on an anti-depressant, then they must be bipolar. It’s entirely too much of a cinch. The data is rendered meaningless by the assumption that any adverse reactions can be blamed on mental illness as if the drugs were magically unable to cause mental disturbance in a person who is already disturbed or just didn’t know they were disturbed until they took an antidepressant.
Wiley,
David Healy makes your point as well, in books, blogs, and in person. He did a “normal” study with SSRIs and also talks about a study with Zoloft that people never published where the phenomena you describe happened. I don’t have the reference handy, but I’ll try to find it. Your point also fits withdrawal. As Alto has pointed out, there are lots of people Dx’d as mentally ill and treated with Drug B when the real problem is withdrawal from Drug A.
Just about every day, someone new comes on my site talking about terrible “dark thoughts” while either ramping up on an antidepressant or coming off. It’s not that infrequent an adverse event! And surely antithetical to the benefit these drugs are supposed to deliver.
Yes… and when we consider the benefits of SSRIs— how are any of the risks justified? No rational person would agree to take these drugs. No parent would jeopardize this child … IF the facts, the simple truth were told.
When I look at Dr. Mickey’s colorful graphs, I am not so much drawn into the complexity of his visual, but with the simplicity of the artistry that seems to say:
“Oh! What a tangled web we weave… when first we practice to deceive”!