In the last post [eyes wide shut open I…], I left off with the results from this study[An Open-Label Comparison of Duloxetine to Other Alternatives for the Management of Diabetic Peripheral Neuropathic Pain]. I’m not going to attempt to summarize all that’s posted [it’s easily accessed with 1-click here]. But here’s a sample, the posting for the Primary Outcome Measure – "Mean Change From Baseline to 12 Weeks in Weekly Mean of Daily 24 Hour Average Pain Score, Pregabalin Compared With Duloxetine."
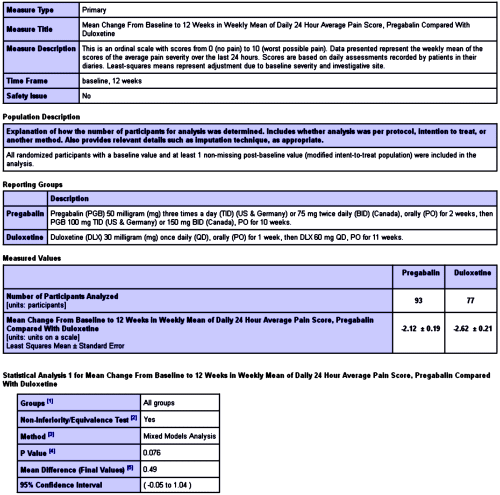
It’s pretty comprehensive. And it goes on and on:

I’m not complaining here. This kind of result reporting surely would’ve deterred Sally Laden and the authors of Study 329 from publishing their Paxil Study in such a deceitful way [or at all]. But, for once, I have a sympathetic thought for the pharmaceutical manufacturers. Looking at all this information so nicely formatted, I can understand why they give them a year to post it, and and at least some of the reason why they are so regularly delinquent [bring on the hoops! [at least the ones that matter]…]:
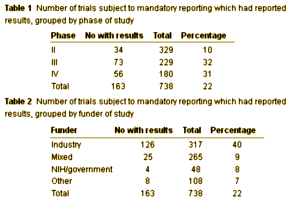
The ClinicalTrials.gov results database was launched in September 2008 to implement Section 801 of the Food and Drug Administration Amendments Act of 2007 (FDAAA 801), which requires the submission of "basic results" for certain clinical trials, generally not later than one year after the Completion Date (see Primary Completion Date data element on ClinicalTrials.gov.) Submission of adverse event information was optional when the results database was released and became required in September 2009. Results information for registered and completed studies is submitted by the study sponsor or principal investigator in a standard, tabular format without discussions or conclusions. The information is considered summary information and does not include patient-level data. The results information that is submitted includes the following:
ClinicalTrials.gov staff review results submissions to ensure that they are clear and informative prior to posting to the Web site. However, ClinicalTrials.gov cannot ensure scientific accuracy. Data providers are responsible for ensuring that submitted information is accurate and complete.
-
I’m opposed to senseless bureaucracy. If they’re going to be required to publish their data, I want to make it as easy as possible for them to do it. The format above doesn’t look easy.
-
This is not really raw data ["The information is considered summary information and does not include patient-level data."]. It’s Means and Standard Deviations, Statistical Comparisons. Those are certainly interesting and standard fare, but frankly, I want to see the individual subject data. I trust it more, and it would be required if, for example, I wanted to do a meta-analysis [or check somebody else’s meta-analysis]. I don’t trust them not to find a way to hide behind the stats just like they’ve done for years.
-
I don’t want to wait. If I read an article that feels jury-rigged, I want to go to the primary data immediately to see if I’m right, or just being too hypervigilant. And any teacher will tell you that you get students to hand in their work before you turn in their grades or give them credit. That’s how you assure that the dog won’t eat their homework. That why I want the results posted as a requirement for either consideration for publication or application for FDA Approval. I want peer reviewers to have access if they want to take a look. I want it there for critical readers to take a look. I want to take a look…
Sorry, the comment form is closed at this time.