Eli Lilly & Co.’s solanezumab and Roche Holding AG’s gantenerumab were selected for a long-term Alzheimer’s trial run by Washington University at St. Louis scientists seeking to block the disease’s symptoms. The experimental medicines will be tested in a trial called DIAN TU involving 160 people with a mutation that guarantees they will develop Alzheimer’s at an early age, perhaps as young as 30, the university said in a statement Wednesday. If the trial is successful, it may lead to those drugs being widely used for people who may have a family history of the disease. Solanezumab slowed the decline in some patients with mild Alzheimer’s and provided no benefit to more advanced patients, Indianapolis-based Lilly announced in August. On Oct. 8, an independent group of scientists confirmed the findings.“Trying to prevent Alzheimer’s symptoms from ever occurring is a new strategy,” said John C. Morris, the trial’s principal investigator, in Wednesday’s statement. That will be the goal of the research, he said. Lilly and Roche, based in Basel, Switzerland, have agreed to make their treatments available for the study at no cost, and will support the trial with grants. Another $4.2 million has been given to the investigation from the Alzheimer’s Association, an advocacy group based in Chicago. The trial will be conducted by Washington University’s unit of the Dominantly Inherited Alzheimer’s Network, or DIAN, an international group of scientists studying rare genetic mutations that cause the disease. The information gathered in these patients may enable better treatment for all dementia patients.
Roche’s gantenerumab binds to clumps of beta amyloid, a protein thought to cause the disease. The drug is thought to help remove the clumps from the brain, and is in a mid- to late-stage company trial that started in 2010. Lilly’s solanezumab removes the beta amyloid before it forms plaques. Another Lilly drug, called a beta secretase inhibitor, is in testing by the company and may be included in the university research as well, according to Wednesday’s statement. That drug is thought to work by reducing the amount of beta amyloid produced in the brain. The trial, which may start early next year, will test the medicines in inherited early-onset Alzheimer’s patients. The researchers will see if the treatments can prevent the loss of brain function.
Clinical and biomarker changes in dominantly inherited Alzheimer disease
by Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC; and the Dominantly Inherited Alzheimer Network.
New England Journal of Medicine. 2012 367[9]:795-804.
BACKGROUND: The order and magnitude of pathologic processes in Alzheimer’s disease are not well understood, partly because the disease develops over many years. Autosomal dominant Alzheimer’s disease has a predictable age at onset and provides an opportunity to determine the sequence and magnitude of pathologic changes that culminate in symptomatic disease. METHODS: In this prospective, longitudinal study, we analyzed data from 128 participants who underwent baseline clinical and cognitive assessments, brain imaging, and cerebrospinal fluid (CSF) and blood tests. We used the participant’s age at baseline assessment and the parent’s age at the onset of symptoms of Alzheimer‘s disease to calculate the estimated years from expected symptom onset (age of the participant minus parent’s age at symptom onset). We conducted cross-sectional analyses of baseline data in relation to estimated years from expected symptom onset in order to determine the relative order and magnitude of pathophysiological changes.
RESULTS: Concentrations of amyloid-beta (Aβ)(42) in the CSF appeared to decline 25 years before expected symptom onset. Aβ deposition, as measured by positron-emission tomography with the use of Pittsburgh compound B, was detected 15 years before expected symptom onset. Increased concentrations of tau protein in the CSF and an increase in brain atrophy were detected 15 years before expected symptom onset. Cerebral hypometabolism and impaired episodic memory were observed 10 years before expected symptom onset. Global cognitive impairment, as measured by the Mini-Mental State Examination and the Clinical Dementia Rating scale, was detected 5 years before expected symptom onset, and patients met diagnostic criteria for dementia at an average of 3 years after expected symptom onset.
CONCLUSIONS: We found that autosomal dominant Alzheimer’s disease was associated with a series of pathophysiological changes over decades in CSF biochemical markers of Alzheimer’s disease, brain amyloid deposition, and brain metabolism as well as progressive cognitive impairment. Our results require confirmation with the use of longitudinal data and may not apply to patients with sporadic Alzheimer’s disease.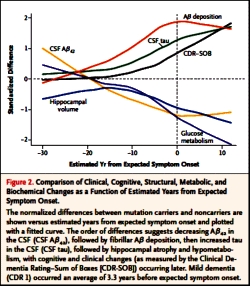
the words and actions seem like the same old market hype
pharma stock prices depend on perceived pipeline prospects
i’ll take your word for it that they seem to be on the track of something
What I mean by “on to something” is that the line of thinking is rational, as opposed to the “let’s try this” approach [“let’s try Atypicals to augment Antidepressants”]. They have a defined disorder [dominantly inherited Alzheimers], a pathology [beta-amyloid], a progression [above], and a treatment strategy [monoclonal antibodies]. But they are in such a rush to market that they’re likely to miss “blind alleys.” Who knows if “clean-em-up” monclonal antibodies are the right treatment strategy, but there are now 10+ candidates chasing that rainbow. They’re so busy desperately racing to market [“translating”] that they don’t have time to think. It’s telling that a failed solanezumab study got reframed just days before they announce its inclusion in another mega-trial. My guess is that there is a correct treatment strategy in this situation but there’s yet to have been the “watson and crick” moment[s] that get[s] them there. Take a look at the “hypothesis factory” on the Alzheimer’s Research site…
Sure they’re excited! Look at how proton pump inhibitor prescriptions skyrocketed.
Here’s a drug for a very narrow indication that got generalized to just about everybody.
There’s lots of room for Alzheimer’s prevention! This is a gold rush that’s going to make 20 years and billions of dollars of psych drug sales look like peanuts.
And if the drugs cause decrease in functioning — how handy that subclinicial pre-existing Alzheimer’s might be responsible for adverse effects.
Note to self: Add to advance directive — no Alzheimer’s drugs. Assisted suicide preferred.