Washington Post
By Peter Whoriskey
November 24, 2012
Blocking biasIn the wake of controversies arising around Vioxx, Avandia and Celebrex, many in the medical world have sought ways to ensure that drug research is free of commercial bias. One of the leading proposals would be to compel drug companies to release all of the data from trials of drugs that are on the market.Over the summer, the European Medicines Agency — the continent’s counterpart to the FDA — said it will move toward requiring the release of all such data. Glaxo, too, has said it is preparing for such a release, though other companies have yet to follow suit. “Since 2004, we have posted summaries of all our clinical trial results on our Web site for the world to see,” Glaxo said in a statement. “All of these actions speak to the degree of commitment we have to be open with our research so there can be more understanding, and hopefully credibility, in what we are doing.”
Such transparency about industry-sponsored trials would not eliminate the ability of companies to avoid unflattering studies, or to hire like-minded researchers, or to design research that gives only positive views of their products. But if such measures are carried out across the industry — and there is no sign at this point that they will be — independent researchers could analyze the data from trials and come to their own conclusions.
Many believe drug companies should feel obliged to share such information. “If you have the privilege of selling a drug, in return should come the responsibility to share everything you know about the drug,” said Harlan Krumholz, a professor of medicine at Yale and a leading advocate of data access. “This is not about doing gotcha with industry. It’s about how to restore trust.”
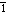
 s]
s]-noun
mental tendency or inclination, especially an irrational preference or prejudice
Origin: 1520–30; Middle French biais oblique
From nearly the beginning, Glaxo scientists confronted signs of potential heart dangers in Avandia. In 2000, about a year after the drug’s approval, a small internal study suggested that Avandia might raise “bad” cholesterol levels more than a competitor.The company considered sponsoring a full-blown trial to weigh the issue, but before it did, scientists conducted a “risk/benefit” analysis — not to calculate the risks and benefits of the drug to patients but to see whether a full-blown trial could harm the drug’s reputation.
When that analysis showed a sign of danger — Avandia raised bad cholesterol levels more than the competitor — the company decided to drop the subject. “The study results support a ‘no-go’ decision,” the internal report concluded, meaning that a full trial would not be conducted.
The researchers even warned one another against sharing the results of the preliminary study. “Per Sr. Mgmt request, these data should not see the light of day to anyone outside of GSK,” said an internal e-mail that was widely reported after it turned up in the Senate investigation…
In approving Avandia, the FDA had asked the company to conduct a trial, known by its acronym ADOPT, to look into the drug’s safety, including “cardiovascular events.” As is common practice, the company arranged for a group of experts — mostly academics — to form a steering committee to guide and publish the experiment. Four of the 11 committee members were Glaxo employees. The other seven reported serving as paid consultants or had other financial connections to the company.The trial would involve more than 4,000 diabetic patients. About one-third would be given Avandia, the rest one of two older, commonly used drugs. But as the FDA later noted, the ADOPT trial was not really designed to assess heart risks.
For one thing, it excluded people most at risk of heart trouble, making it harder to spot a problem. Moreover, investigators did not have a group of doctors validate reports of heart attacks, as is customary because they can be difficult to detect. Finally, about 40 percent of patients dropped out of the trial…
Having worked up a froth pondering the word bias, I reread Whoriskey’s article. When I read GSK saying, “Since 2004, we have posted summaries of all our clinical trial results on our Web site for the world to see. All of these actions speak to the degree of commitment we have to be open with our research so there can be more understanding, and hopefully credibility, in what we are doing.”, I thought of my weekend poring through one of those very GSK 2004 summaries [paxil study 352 revisited…]. This weekend, I didn’t think of commitment, I thought of Hermione in the Harry Potter series waving her wand saying "Obscuratum!" I was way underwhelmed with "open with our research."
And when I read "… the company arranged for a group of experts — mostly academics — to form a steering committee to guide and publish the experiment. Four of the 11 committee members were Glaxo employees. The other seven reported serving as paid consultants or had other financial connections to the company", I thought of their recent announcement [need to hear more…] that they would make raw data available contingent on approval by a panel – "The company intends to name an independent panel of experts to review requests submitted by researchers." Their track record with named panels isn’t reassuring to me.
Bias is a versatile word, with a range of connotations. Not all of them are so innocent as a personal predisposition to a point of view. In science, bias means loss of a disinterested posture – which is exactly what is called for in experimental medicine. The inherent conflict in modern drug development is that corporations are anything but disinterested, and so the experimercials they design will contain biases, as the Avandia story illustrates. When I went to the OED this morning there was an apposite 1669 quote from Dryden: “Your bowl must be well byassed to come in.” Just as the bias built into a bowling ball is deliberate, so it is with experimercial drug trials. They are rigged to favor the candidate new drug. The bias may be subtle but it is definitely there, again as the Avandia story makes clear. We could say the same for Glaxo study 329 and Glaxo study 352. The loss of a disinterested posture is the failing of KOLs who become cheerleaders for their corporate paymasters. And as the sociobiologists will tell us, if you wish to deceive others then it helps first to deceive yourself about your bias and your intentions.
Then there is a whole other level of deceit that you are right to say goes beyond bias into the territory of misrepresentation or even fraud. Things like HARKing or changing endpoints or suppressing inconvenient toxicity data or even flat out lying about p values. We don’t have to look far for examples in psychiatry.