 The flooding of the literature with industry sponsored articles whose primary function was advertisement is well known to most of us. GlaxoSithKline’s Paxil studies authored by Scientific Therapeutic Information [Sally Laden] and Janssen’s series of Risperdal articles managed by Excerpta Medica come immediately to mind [Rothman Report]. In the complaint in a recent suit filed against Pfizer [oh look…], there’s a reference that I hadn’t reviewed before about an extensive campaign by the Current Medical Direction [CMD] company to promote Zoloft. Recall that Zoloft was the third SSRI on the scene [following Prozac and Celexa], definitely in the "me too" position. I’d heard about this article, but wasn’t prepared for the magnitude of the campaign – the majority of the articles were from this communications company:
The flooding of the literature with industry sponsored articles whose primary function was advertisement is well known to most of us. GlaxoSithKline’s Paxil studies authored by Scientific Therapeutic Information [Sally Laden] and Janssen’s series of Risperdal articles managed by Excerpta Medica come immediately to mind [Rothman Report]. In the complaint in a recent suit filed against Pfizer [oh look…], there’s a reference that I hadn’t reviewed before about an extensive campaign by the Current Medical Direction [CMD] company to promote Zoloft. Recall that Zoloft was the third SSRI on the scene [following Prozac and Celexa], definitely in the "me too" position. I’d heard about this article, but wasn’t prepared for the magnitude of the campaign – the majority of the articles were from this communications company:Interface between authorship, industry and science in the domain of therapeutics
by Healy D and Cattell D.
British Journal of Psychiatry. 2003 183:22-27.
[full text on-line]
BACKGROUND: Changes in the character of medical authorship. Aims To compare the impact of industry-linked and non-industry linked articles.METHOD: We compared articles on sertraline being coordinated by a medical writing agency with articles not coordinated in this way. We calculated numbers of Medline-listed articles per author, journal impact factors, literature profiles and citation rates of both sets of articles.RESULTS: Non-agency-linked articles on sertraline had an average of 2.95 authors per article, a mean length of 3.4 pages, a mean Medline listing of 37 articles per author [95% CI 27-47] and a mean literature profile of 283 per article [95% CI 130-435]. Agency-linked articles on sertraline had an average of 6.6 authors per article, a mean length of 10.7 pages, a mean Medline listing of 70 articles per author [95% CI 62-79] and a mean literature profile of 1839 per article [95% CI 1076-2602]. The citation rate for agency articles was 20.2 [95% CI 13.4-27.0] and for non-agency articles it was 3.7 [95% CI 3.3-8.1].CONCLUSIONS: The literature profiles and citation rates of industry-linked and non-industry-linked articles differ. The emerging style of authorship in industry-linked articles can deliver good-quality articles, but it raises concerns for the scientific base of therapeutics.
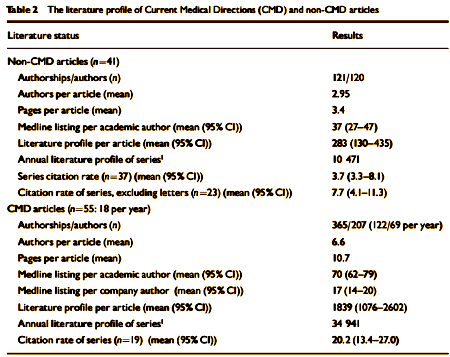
In the article’s text it tells that many of these articles were drafted with authors "to be determined" as was the case with the Janssen Excepta Medica articles and the GSK Paxil 352. Only two of the 55 CMD articles acknowledged writing support from individuals not listed as authors. More disturbing than the sheer number of professionally written, industry managed articles was the evidence of data distortion:
The second issue relates to the correspondence between published articles and raw data. The current CMD series throws up issues of concern in this area. First, one study in this series had one patient on sertraline who committed suicide, and three others on sertraline who reported increasing suicidal ideation necessitating treatment discontinuation, in contrast to just one case of emergent suicidality on a comparable drug and no problems on placebo. There is no reference to these data in the final published article. Second, of the six published paediatric psychopharmacology CMD articles, only one article mentions one suicidal act. There were in fact six suicidal acts on sertraline and three further cases of suicidality in the subject group from which these articles come, including four suicidal acts in 44 patients with depression given sertraline, which is a rate of 9% (Pfizer Expert Report, 1997). The effects of sertraline in paediatric depression were outlined by Alderman et al (1998), who reported only the adverse events that occurred in more than 10% of patients.
Most of us are now used to knowing about the ghost-writing and professional management of these articles in our peer-reviewed literature. But if you are reading this, I suspect you are in the minority with such insider information. When I mention it to friends in Atlanta, they say something like "I’m not surprised," but it’s clearly new information. Had I read this Healy and Cattell piece in 2003, the year I retired, I wouldn’t have had a clue about any of this, not at all.
 On a related note, the suit where I found this reference is kind of unique [Laura A. Plumlee et. al. v. Pfizer]. It basically alleges that Zoloft was a creation of false advertisement, that it was an ineffective antidepressant, and they want their money back with damages ["they" being everybody that took it]. That seemed kind of radical at first glance, but reading it through, it deserves a more thorough review. They’ve certainly cast a wide net:
On a related note, the suit where I found this reference is kind of unique [Laura A. Plumlee et. al. v. Pfizer]. It basically alleges that Zoloft was a creation of false advertisement, that it was an ineffective antidepressant, and they want their money back with damages ["they" being everybody that took it]. That seemed kind of radical at first glance, but reading it through, it deserves a more thorough review. They’ve certainly cast a wide net: WHEREFORE, Plaintiff, individually and on behalf of the California Consumer Class described herein, prays for the following relief:
a. Find that this action satisfies the prerequisites for maintenance of a class action pursuant to Federal Rules of Evidence 23(a), (b)(1), (b )(2), and (c)(4), and certify a Nationwide Issue and Injunctive Class described more fully herein.
b. Designate Plaintiff as a representative for the Nationwide Issue and Injunctive Class and her counsel as Class counsel.
c. Issue a judgment against Pfizer that:
i. Declares the following:
1. Zoloft’s drug label. between 1991 to the present, was misleading or deceptive because it did not contain material information about Zoloft’s efficacy, to wit, the drug label failed to provide information about the majority of clinical trials demonstrating that Zoloft was no more effective than placebo in treating depression and specify the marginal benefit to treating depression observed in the two clinical trials purporting to show Zoloft’s efficacy;
2. Pfizer intentionally, deliberately, or recklessly created and distributed Zoloft’s misleading drug label with regard to its efficacy description for depression.
ii. Permanently enjoins Pfizer from continuing to sell or market Zoloft with its current drug label and directing Pfizer to seek FDA approval of a new label that properly discloses Zoloft’s efficacy.
iii. Grants Plaintiff and the Nationwide Issue and Injunctive Class reasonable attorneys’ fees and costs of suit; and
iv. Grants Plaintiff and the Nationwide Issue and Injunctive Class such other and further relief as the Court deems just and proper under the circumstances.
Sorry, the comment form is closed at this time.