by Sidney M. WolfeBritish Medical Journal. 2013 347:f7507.Are criminal and civil penalties of hundreds of millions of dollars an important deterrent to law breaking by international drug companies? Further, would external monitoring in the form of US government mandated corporate integrity agreements [CIA] to prevent recurrences of such illegal activities, lasting five years after being signed, be an additional deterrent? Yes in both cases, but only if the size of the penalties outweighed the companies’ gains while violating the laws and only if enforcement of the CIAs were effective. Unfortunately, neither is the case. This evaluation is based on the recent, sharp escalation in the frequency with which many giant multinational drug companies repeatedly engage in illegal criminal and civil activity after previously paying enormous fines and despite monitoring under CIAs. It seems that for some companies, commission of such criminal and civil violations has become part of their business models.
[see a sad tale… for Public Citizen’s September 2012 report also below]
An analysis of several of the repeat civil payments and criminal penalties by GSK and Pfizer based on publicly available government documents illustrates the failure of such large penalties and the parallel failure of the accompanying CIAs to curb such behavior.
Profits in 2012 alone for GSK amounted to $7.7bn and, for Pfizer, $10bn, both greater than the entire amount the companies paid to the government during the 21 plus year interval from January 1991 to mid-2012. These escalating patterns of repeated criminal violations and civil settlements to resolve serious allegations of civil lawlessness hardly bespeak corporate integrity for GSK, Pfizer, or the many other companies who are also repeat offenders. We are forced to conclude that neither the current level of penalties nor corporate integrity agreements are effective and that there is a pathological lack of corporate integrity in many drug companies.
There’s a really big question on the table these days. The misbehavior of the Pharmaceutical Industry is becoming public – the stuff of legends: jury-rigged ghost-written clinical trial reports, marketing off-label prescribing, direct payola to physicians. The sins have been industry-wide, focused primarily on lifestyle and psychoactive drugs, but spilling over into most areas of medicine. Recently, they’ve made a number of initiatives aimed at clearing up their public image: signing on to the AllTrials petition; tempering their stand on data availability; discontinuing paying doctor speakers. Are these trojan horses? wolves in sheep’s clothing? signs of a change of heart? or an admission of defeat? There’s a non-dichotomous choice in here as well, signs of internal differences within these companies?
Sidney Wolfe and the Public Citizen give us a splash of cold water with their 2012 Report and this update. Rather than obsess about what the Pharmaceutical Companies say, they’re looking at what they actually do. Last year’s report documented the rapid rise of successful suits against them – a trend that persists. This article focuses on two major players, the largest in the UK, GSK, and the US, Pfizer. In spite of record fines and signing Corporate Integrity Agreements, they just barrel ahead seemingly unmoved by either penalty.
"Unless we can find a solution to the commercial incompetence problem, we have to recognize that the pharmaceutical industry has an irreducible conflict of interest in relation to the way it represents its drugs, in science and in marketing. And unless we can resolve this in a way that is more in the public interest and in patients’ interest, I would argue that drug companies should not be allowed to evaluate their own products."
New York Timesby Marcia AngellDecember 16, 2013The pharmaceutical industry has responsibility for evaluating its own products. Even on the face of it, that makes no sense. How can investor-owned companies whose responsibility to shareholders is to maximize profits be expected to evaluate their drugs objectively? Yet, even though drug companies make products that profoundly affect people’s lives, they are given great control over whether the drugs are considered safe and effective. This is how it works. Drug companies sponsor the clinical trials that must be submitted to the F.D.A. to get approval to sell prescription drugs. The problem is that they can design those trials to make a favorable outcome much more likely. For example, they can test a drug in young people, even though it will be used in older people, because young people are less likely to have side-effects that indicate safety problems.
A company may sponsor several clinical trials of a drug in the hopes that one or two will yield statistically favorable results, even though the clinical usefulness might be negligible and all the other trials might show no effect. The F.D.A. might then approve the drug on the basis of just a couple of trials. The drug company publishes the favorable trials and markets the drug heavily on the basis of those trials, while all the negative studies remain buried at the F.D.A, without any publicity whatsoever. That is how “breakthrough” drugs are born. In this way, both physicians and the public come to believe that prescription drugs are much safer and more effective than they are.
Moreover, the part of the F.D.A. that reviews new drugs is paid “user fees” by the companies whose drugs they are evaluating – a clear conflict of interest. The fees, paid for each drug, provide most of the salaries of the people who work in that section of the F.D.A. There is therefore a strong incentive to approve new drug applications rapidly — since that is faster than denying approval. In addition, many members of the agency’s outside advisory committees have personal financial ties to drug companies. I believe the “user” of the F.D.A. is the public, and that the agency should be entirely supported by the public it presumably serves. Advisory committees should consist only of academic experts without ties to industry.
Also, rather than having the companies that profit from the drugs testing them, an Institute for Prescription Drug Trials should be established within the National Institutes of Health to oversee clinical trials, which should be conducted by investigators without conflicts of interest. Prescription drugs are too important to be evaluated by the companies that make them.
 As a boy, one summer I was sent to something called Vacation Bible School. The teacher explained that in the Old Testament, people believed in "An eye for an eye and a tooth for a tooth." I didn’t exactly know what she meant, but I got the gist of it. Then she said that Jesus came along and said, "Turn the other cheek." I sort of got that too. My main concern at the time was the bully who was torturing me every day at recess, and I was unimpressed that either option worked, kept me from being beaten up. I had tried them both. Not long after that, I read one of those little orange biographies about President Teddy Roosevelt who said, "walk softly and carry a big stick." Now there was some advice I could get with. And while sneaking up and whacking that kid in the head with a limb didn’t do much to immediately improve my lot [when the teacher called my parents], I can report a long term benefit [early retirement from Bible School]. That may be my only direct application of the Roosevelt principle, but its effectiveness persists in memory. As a boy, one summer I was sent to something called Vacation Bible School. The teacher explained that in the Old Testament, people believed in "An eye for an eye and a tooth for a tooth." I didn’t exactly know what she meant, but I got the gist of it. Then she said that Jesus came along and said, "Turn the other cheek." I sort of got that too. My main concern at the time was the bully who was torturing me every day at recess, and I was unimpressed that either option worked, kept me from being beaten up. I had tried them both. Not long after that, I read one of those little orange biographies about President Teddy Roosevelt who said, "walk softly and carry a big stick." Now there was some advice I could get with. And while sneaking up and whacking that kid in the head with a limb didn’t do much to immediately improve my lot [when the teacher called my parents], I can report a long term benefit [early retirement from Bible School]. That may be my only direct application of the Roosevelt principle, but its effectiveness persists in memory. |
J&J’s Alex Gorsky may have gone to West Point and may be America’s oldest Boy Scout [shoveling…] and GSK’s Andrew Witty might be the number one peer of the realm, a modern Lancelot, but the megalithic pharmaceutical companies are in business to make money. You can bet that if they have their hands on a clinical trial like Paxil Study 329 that has a weak but not quite significant signal, somebody is going to fall to temptation. The same with bribes. The stakes are just too high and it has happened too regularly to ignore. It’s in the nature of those companies. It’s time to stop moralizing about that and get us a big stick. They’re businessmen. And no matter how often it’s said, it’s still true. It’s just "the cost of doing business."
As we have said endlessly, true health care reform will not occur until the leaders of large health care organizations are made accountable for their actions, and are prevented from becoming amazingly rich while their organizations repeatedly commit unethical or illegal acts that harm patients’ and the public’s health.
These are large, lucrative corporations that spend more money and time on these matters than on any other aspect of their business, including research. Even when backed into a corner, they settle and don’t admit to wrong-doing. I think Teddy would be proud of the directions suggested here. The best case scenario is, First, make no compromises in the current push for data transparency up front. These companies have behaved outrageously and deserve no concessions for any reason. Data secrecy is like segregation or apartheid, unjustifiable on any count. Second, I say pursue R.I.C.O. if at all possible in every prosecution. This is organized crime by any definition, and that’s why we have R.I.C.O. It’s meant, among other things, to prosecute people, criminals. We need to put names and faces on these cases. You can’t settle out of a R.I.C.O. criminal prosecution of a person. I suggest the complicit KOL physicians are criminals and their names belong on these suits too. And finally, if it continues, do the Godlee/Angell thing – take clinical trials away from them altogether. These companies are big, but not too big to fail. If I had to be a doctor using generic drugs until a new more reasonable pharmaceutical industry emerged, I’d be okay with that. I practice psychiatry still, and prescribe only generics because of my patient’s resources. We are doing absolutely fine. I fill in with my old Internist identity in a pinch, and we do fine there too.
One thing that I think might be an essential ingredient – an agency, existing or new, that is in charge of this problem. These cases show up in a variety of courts, jurisdictions, interfacing with government medical programs of different kinds. It’s a big enough problem to deserve a task force or an agency of its own, particularly with the government’s increasing [and necessary] involvement in the healthcare industry. I’m not a big government type and believe medicine should be self regulating, but this problem is so out of hand that it needs a central coordinating function to apply the necessary pressure sequentially until it’s under control.
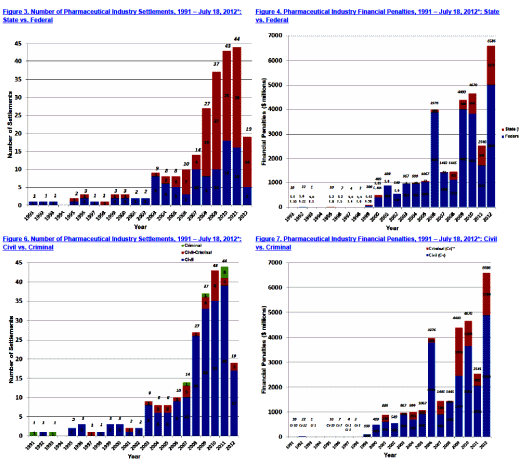
Nice big picture summary. Laid out like that it is perfectly preposterous that this is how it works.
This administration is busting doctors and institutions involved in Medicare/Medicaid fraud and putting billions of stolen dollars back where they belong. It’s time to start pressing criminal charges for fraudulent practices in pharmaceutical testing and peddling. There is no way the industry can ever compensate for all the damage it’s done, but their ability to continue these fraudulent, money grubbing practices could be cut to the nub by Marcia Angells suggestions. They could also be leveled fines so steep that they would not dare not continue on this path. Let the stock holders feel the pain, they have a moral obligation not to profit from fraud whether they care or not.