 This morning’s thermometer says that we’re finally starting to thaw out down here a bit. Time for a real blog with graphs and numbers. Within the few years after the introduction of the Selective Serotonin Reuptake Inhibitor [SSRI] class of antidepressants, their association with suicidal ideation, suicide attempts, or suicides became the question of the hour based on case reports. There was an FDA Hearing in 1991 that didn’t find a signal sufficient for action. But by the next FDA Inquiry in 2004, meta-analysis of Clinical Trials was added to the case reports, and the FDA concluded that a signal justified adding a Black Box Warning to all antidepressants for adolescents, later extended to young adults. Throughout this period, investigators have also attempted to examine large practice databases to clarify this relationship.
This morning’s thermometer says that we’re finally starting to thaw out down here a bit. Time for a real blog with graphs and numbers. Within the few years after the introduction of the Selective Serotonin Reuptake Inhibitor [SSRI] class of antidepressants, their association with suicidal ideation, suicide attempts, or suicides became the question of the hour based on case reports. There was an FDA Hearing in 1991 that didn’t find a signal sufficient for action. But by the next FDA Inquiry in 2004, meta-analysis of Clinical Trials was added to the case reports, and the FDA concluded that a signal justified adding a Black Box Warning to all antidepressants for adolescents, later extended to young adults. Throughout this period, investigators have also attempted to examine large practice databases to clarify this relationship.
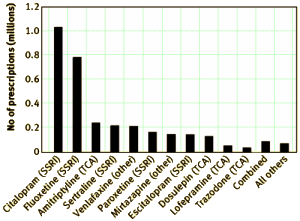
by Carol Coupland, Trevor Hill, Richard Morriss, Antony Arthur, Michael Moore, and Julia Hippisley-CoxBritish Medical Journal. 2015;350:h51.
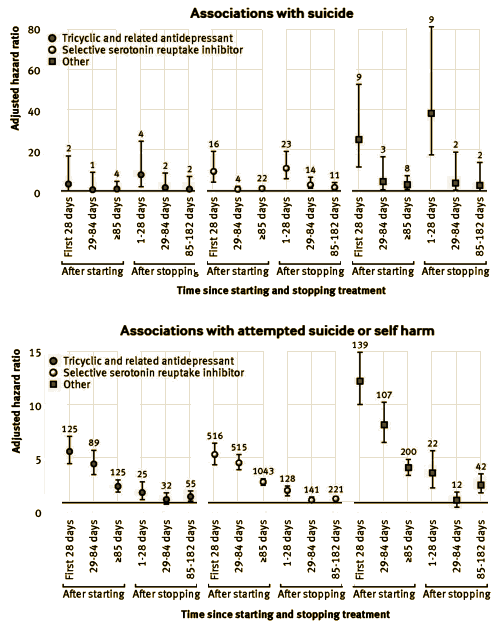
Objective: To assess the associations between different antidepressant treatments and the rates of suicide and attempted suicide or self harm in people with depression.Design: Cohort study. Setting Patients registered with UK general practices contributing data to the QResearch database. Participants 238 963 patients aged 20 to 64 years with a first diagnosis of depression between 1 January 2000 and 31 July 2011, followed up until 1 August 2012.Exposures: Antidepressant class [tricyclic and related antidepressants, selective serotonin reuptake inhibitors, other antidepressants], dose, and duration of use, and commonly prescribed individual antidepressant drugs. Cox proportional hazards models were used to calculate hazard ratios adjusting for potential confounding variables.Main outcome measures: Suicide and attempted suicide or self harm during follow-up.Results: During follow-up, 87.7% [n=209 476] of the cohort received one or more prescriptions for antidepressants. The median duration of treatment was 221 days [interquartile range 79–590 days]. During the first five years of follow-up 198 cases of suicide and 5243 cases of attempted suicide or self harm occurred. The difference in suicide rates during periods of treatment with tricyclic and related antidepressants compared with selective serotonin reuptake inhibitors was not significant [adjusted hazard ratio 0.84, 95% confidence interval 0.47 to 1.50], but the suicide rate was significantly increased during periods of treatment with other antidepressants [2.64, 1.74 to 3.99]. The hazard ratio for suicide was significantly increased for mirtazapine compared with citalopram [3.70, 2.00 to 6.84]. Absolute risks of suicide over one year ranged from 0.02% for amitriptyline to 0.19% for mirtazapine. There was no significant difference in the rate of attempted suicide or self harm with tricyclic antidepressants [0.96, 0.87 to 1.08] compared with selective serotonin reuptake inhibitors, but the rate of attempted suicide or self harm was significantly higher for other antidepressants [1.80, 1.61 to 2.00]. The adjusted hazard ratios for attempted suicide or self harm were significantly increased for three of the most commonly prescribed drugs compared with citalopram: venlafaxine [1.85, 1.61 to 2.13], trazodone [1.73, 1.26 to 2.37], and mirtazapine [1.70, 1.44 to 2.02], and significantly reduced for amitriptyline [0.71, 0.59 to 0.85]. The absolute risks of attempted suicide or self harm over one year ranged from 1.02% for amitriptyline to 2.96% for venlafaxine. Rates were highest in the first 28 days after starting treatment and remained increased in the first 28 days after stopping treatment.Conclusion: Rates of suicide and attempted suicide or self harm were similar during periods of treatment with selective serotonin reuptake inhibitors and tricyclic and related antidepressants. Mirtazapine, venlafaxine, and trazodone were associated with the highest rates of suicide and attempted suicide or self harm, but the number of suicide events was small leading to imprecise estimates. As this is an observational study the findings may reflect indication biases and residual confounding from severity of depression and differing characteristics of patients prescribed these drugs. The increased rates in the first 28 days of starting and stopping antidepressants emphasize the need for careful monitoring of patients during these periods.
Supplementary Data: «link»
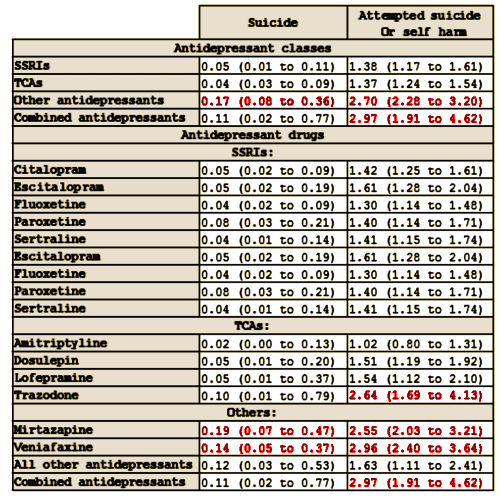
The suicide rates in our study cohort [43 per 100 000 in men and 9 per 100 000 in women] are higher than those in the general population in England [three year average rates of 12.4 per 100 000 in men and 3.7 per 100 000 in women for 2010-12]. Larger differences than this might be expected since our rates are in patients with a diagnosis of depression rather than in the general population. Studies showing greater differences, however, have tended to be in secondary care settings, where patients have more severe depression. These results are consistent with those of our previous cohort study in older people with depression, which found that trazodone, mirtazapine, and venlafaxine were associated with the highest rates of attempted suicide or self harm in people aged 65 or more.
Conclusions:This study has found that rates of suicide and self harm were similar during periods of treatment with selective serotonin reuptake inhibitors and tricyclic antidepressants, but were higher for the group of other antidepressant drugs, with mirtazapine, venlafaxine, and trazodone being associated with the highest risks. The number of suicide events was small so the results for suicide should be interpreted with caution. Rates tended to be highest in the first 28 days after starting treatment and remained increased in the first 28 days after stopping treatment. These findings are of associations rather than causal effects and are particularly susceptible to confounding by indication, channelling bias, and residual confounding; further research is needed to confirm them. The results of this study indicate that patients taking antidepressant drugs should be carefully monitored, especially during early treatment with antidepressants and when stopping treatment.
When Lilly came out with Prozac in the late 1980s they sent an army of sales reps into general practice offices with a branding message: Here is the new way to treat depression; it’s a single dose once a day; it works and there are no side effects worth worrying about; now go back to agonizing about how you can treat hypertension. In its day, this branding message did away with professional concerns about toxicity of antidepressant drugs, did away with academic calls for blood level monitoring, did away with the nuances of assessment and subtype diagnosis, and brushed away the perceived need to monitor patients carefully in the early weeks of treatment. Overall, this branding message dumbed down clinical depression to a mostly trivial condition and removed the brakes from pragmatic prescribing for nonspecific unhappiness. That’s what marketing departments do, and psychiatry allowed them inside the tent. These new data help us to push back against the marketeers.