Back in August, I sent this email out to a number of people
| I’m one of the authors of the RIAT article about Paxil® Study 329 due out soon in the BMJ. In the course of things, I have a question that I find hard to get a definitive consistent answer to, so I decided to write some people who are involved with the issue of Data Transparency and ask directly.
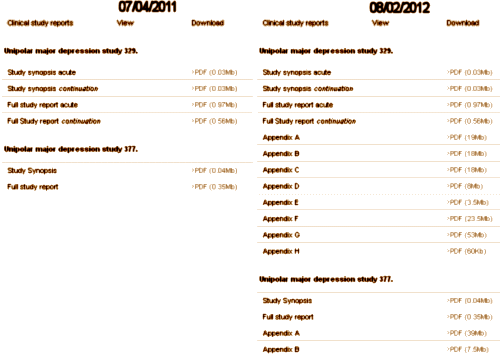
Background: In 2004, GSK settled a suit in New York State brought by Elliot Spitzer, then Attorney General. As part of that settlement, they agreed to post the data from their pediatric studies of Paxil® on a public clinical trial registry. What they actually posted in on the left side of this figure:
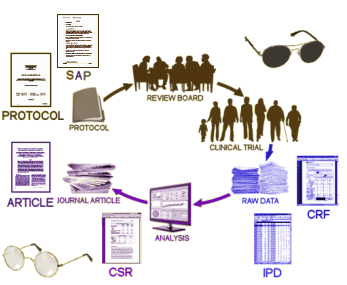
They were the Full Study Report Acute and the Full Study Report Continuation. Neither contained the "data." They were both elaborate and detailed reports that said what the paper itself said. They were of no real use in vetting the paper itsef. If anything they were more "jury-rigged" than the published paper by Keller et al. In discussions, they were referred to as "the CSR." In August 2012, Peter Doshi [of Tamiflu fame] noted the absence of raw data, and contacted the new Attorney General of New York. I don’t know the details of how, but the Appendices appeared on the site that month [on the right side of the figure]. They were the "raw data," and became the nidus for our RIAT article, though we later were given access to an electronic form of those appendices and the CRFs via the secure data portal by applying to GSK. My Question: In discussions of Data Transparency, people often speak of "the CSR" as what will be made available in the various Data Transparency schemes. In our case, the "CSR" was not Data Transparency at all. It was yet another authored version of the published paper with the same manipulations. The later release referred to the actual "raw data" as appendices. My own understanding of the various documents is in this figure:
with "CSR" [clinical study report] meaning the narrative report and "IPD" [individual participant data] meaning the actual "raw data" itself. In our case the "CSR" was no help. I had read its 528 pages repeatedly with no more clarity than I had from the paper [actually, it was more confusing]. The "IPD" in the late-coming appendices was required to accurately "vet" the Clinical Trial. I genuinely find the various discussions of this topic confusing when people use these acronyms. Would you please briefly clarify your understanding of the terms "CSR," "IPD," and "Data Transparency." Is the "IPD" a required part of the "CSR?" What do you envision actually being available in the future? I am obviously worried that this lack of clarity may serve to undermine the important movement for true Data Transparency. Earlier efforts at reform have floundered on just such imprecision… |
I got a number of responses, mostly from people like me who are interested in Data Transparency as a reform movement. But today, I got a response from the European Medicines Agency, people who are actually in the driver’s seat. I’m posting it in its entirety as a reference. My comments to follow after some digestion. I certainly appreciate the detailed response from whomever wrote this:
|
Thank you for your query about the EMA’s understanding of various terms/acronyms that are mentioned in discussions around data transparency. In order to address your questions in a concrete manner we would like to respond to them in the context of one of the “various Data Transparency schemes”: Policy 0070- EMA policy on publication of clinical data for medicinal products for human use.
Through this policy the EMA aims to make available to the public clinical data once the regulatory decision phase has concluded. In the context of this policy the term “data” concerns the documents submitted under the centralised marketing authorisation procedure. For further details on which regulatory procedures are covered by this initiative please see section 2 of Policy 0070.The policy will be implemented in a stepwise manner. In the first phase (currently under implementation) the following 3 types of documents/clinical reports will be published:
I hope that this information clarifies to you the terms used by the EMA.
We note that your questions focus on one of the above types of clinical reports, namely the CSRs. Therefore our answer to “What do you envision actually being available in the future?” is specific to the CSRs. In phase 1 of the policy the CSR narrative along with ONLY three (3) of the Appendices: 16.1.1 (protocol and protocol amendments), 16.1.2 (sample case report form), and 16.1.9 (documentation of statistical methods) will be published proactively. Appendices 16.2.1 to 16.2.8, which can be found under the heading “16.2. PATIENT DATA LISTINGS” as defined in ICH E3, come within the scope of phase 2 of the policy. The first image you refer to in your e-mail does not name the Appendices in a specific manner (A to H). Therefore we are unable to confirm whether Appendixes A to H (from the image) correspond to Appendixes 16.2.1 to 16.2.8 as defined in ICH E3. Based on the details given in your message, we made the assumption that the Appendices you refer to are those listed under “16.2. PATIENT DATA LISTINGS” as defined in ICH E3. For ease of reference, the latest update on the implementation of EMA policy on publication of clinical data for medicinal products for human use (Policy 0070), is available on the following webpage Current status of European Medicines Agency policy on publication of clinical data – Stakeholder webinar. The web page has details of a webinar organised by EMA on the implementation of Policy 0070. The aim of the webinar was to update stakeholders on the progress the Agency has made on the implementation of the policy. The topics covered by the webinar included an explanation of the principles for the submission of redacted clinical reports, the redaction consultation process, as well as guidance on what is and is not considered commercially confidential information and on the anonymisation and redaction of personal data in clinical reports. Yours sincerely,
Stakeholders and Communication Division
|
Sorry, the comment form is closed at this time.