FiercePharmaBy Tracy StatonMarch 29, 2016The FDA doesn’t have to follow the advice of its expert review panels, but it usually does. That’s a standard line in stories about advisory committee votes. Unfortunately for Lundbeck and Takeda, their new Brintellix app is one of the unlucky ones. The agency issued a complete response letter on new labeling that might have given Brintellix a new edge in the crowded antidepressant field. The claim: Brintellix can give a brainpower boost to depressed patients, whose thinking abilities often suffer. Lundbeck and Takeda had scored study data showing that the novel med can help patients think, pay attention, and make decisions – something no other antidepressant had managed to pull off. And the trial didn’t test Brintellix against a placebo, but head-to-head with Eli Lilly’s rival drug Cymbalta.
When an FDA advisory panel met last month to review those data, they voted 8-2 for an approval. European regulators had already approved a new use for Brintellix, to improve cognitive function in depressed adults. The FDA didn’t agree, and that puts Lundbeck and Takeda’s long-term sales projection – $2 billion – in jeopardy. Lundbeck developed the med, and Takeda has U.S. marketing rights under a marketing deal with the Danish drugmaker…
In any case, the hoped-for cognitive claim will be delayed at best, and with just $94 million in 2015 sales, Brintellix hasn’t lived up to early launch expectations. It faces considerable competition in depression, particularly now that a raft of commonly used meds – including Pfizer’s Zoloft, Eli Lilly’s Prozac and GlaxoSmithKline’s Paxil – are now available in cheap generic versions. And with payers requiring patients to try older, less expensive meds before moving to brands, the marketing task for Brintellix has been heavy. After the panel vote last month, one investment firm pointed out that Brintellix would be "the only product on the market with a leaflet on cognitive effects," Alm. Brand wrote in a note to clients. Focusing label language on improvements in thinking, attention and decision-making "means doctors also will," the firm said…
Meanwhile, Lundbeck has been laying off workers and cutting other costs as part of a plan to save 3 billion kronor, or about $445 million, in annual costs by 2017. Most of the cuts were focused on the company’s HQ in Denmark and commercial operations, mainly in Europe.
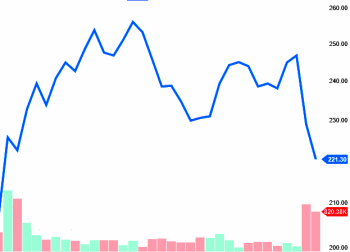
Lundbeck stock from Feb 9 to March 30 [between the two FDA hearings]
During the last month, I’ve talked to a couple of people who mentioned that they’d been started on Brintellix® [given samples]. They both said something like, "It’s a new one. It’s supposed to help you [focus or think] better." or something like that. These were not patients – but the daughter of a friend and a friend of my daughter. I didn’t really register much at the time, but thinking about it now, that had to come from somewhere to the prescribing primary care doctor. I would guess from a detail person [drug rep]. While my anectdote is hardly a scientific survey, it gives me a pause. Whether it’s on a package insert or "a leaflet" doesn’t matter if it’s in the dialog. It’s like the political "talking points" that spread from talk radio host into the general conversation. And the investor guy isn’t wrong when he says, "Focusing label language on improvements in thinking, attention and decision-making ‘means doctors also will,’ the firm said…."
This vortioxetine campaign is pure and simple pharmaceutical bottom fishing, coupled with disease-fragment mongering. Good for the FDA on this one!