Recently, FDA/NIH/clinicaltrials.gov issued their long awaited reforms with something of a media blitz:
- Toward a New Era of Trust and Transparency in Clinical Trials
- Trial Reporting in ClinicalTrials.gov — The Final Rule
- New federal rules target woeful public reporting of clinical trial results
- HHS Issues New Rules To Open Up Data From Clinical Trials
I’m impressed that the N.I.H. and its ClinicalTrials.gov registry under the respective direction of Francis Collins [NIH Director] and Deborah Zarin [ClinicalTrials.gov Director] have done a good job of identifying the problems and clarifying loopholes. Dr. Collins focuses on the government funded studies and Zarin on ClinicalTrials.gov procedures. While I have something to say about their solutions [below], they’ve definitely taken a long look at the sins of the fathers and sent down some credible commandments. However, I can’t say that I was at all reassured by the FDA’s response. If they had a Press Release, I couldn’t find it. It wasn’t mentioned on their FDA blog. Here are the quotes from Robert Califf from the articles above:
From the STAT article [New federal rules target woeful public reporting of clinical trial results]:
And that was all I could find from the FDA. I would cop to a charge of over-reading Califf’s comments, in a New York minute, but it’s even worrisome to me that that’s all I can find to read. Best I can tell, the FDA action will be to piggy-back onto some automated NIH/ClinicalTrials.gov automate[d] compliance check[er]. Otherwise, I hear "don’t worry, they will take care of it." And, by the way, it’s the private sector that needs the over·seeing ["according to Califf, whose agency oversees private sector reporting, and NIH Director Francis Collins, whose agency is responsible for enforcement regarding NIH grant recipients"]. So I’m filing the FDA’s response under "wary" cross-referenced with "uh-oh!" This is how reform begins its decline to the state we’re in already.
And in spite of being impressed that the NIH/ClinicalTrials.gov final rule shows signs of some real effort and investigation, I question their solutions. There are two points in the life cycle of a clinical trial where data is entered into ClinicalTrials.gov – Registration and Results:
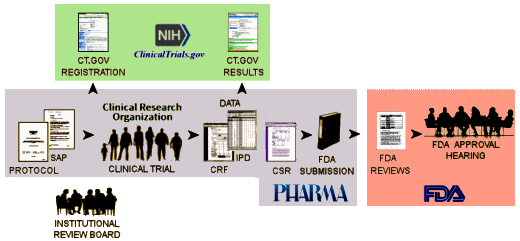
Here’s a shortened version of what the final rule has to offer:
-
Registration [the final rule]:
when – "Submission: Within 21 days after enrollment of the first trial participant. Posting: Generally, within 30 days after submission…"
what – "Descriptive information about the trial: e.g., brief title, study design, primary outcome measure information…" -
Results [the final rule]:
when -"Submission: Standard deadline: Within 12 months after the date of final data collection for the prespecified primary outcome measures (primary completion date)…"
what – "Outcomes and statistical analyses for each primary and secondary outcome measure by treatment group or comparison group, including results of scientifically appropriate statistical analyses performed on these outcomes, if any. Adverse event information: Tables of all anticipated and unanticipated serious adverse events and other adverse events that exceed a 5% frequency threshold within any group"
I consider myself a reasonably well-informed consumer, and I’m disappointed for the following reasons:
-
Registration: Preregistration of the Primary and Secondary Outcome Parameters and the Methods by which they will be analyzed is an essential ingredient of a scientifically conducted clinical trial. Changing outcomes after the study is underway has been a major method used to deceitfully jury-rig the results [see ]. Ergo any effective reform must insure that a certified copy of that a priori Protocol and Statistical Analysis Plan is filed before the trial begins. Filing those things with the Results in case there are amendments is also fine, but does nothing to reassure us that these variables haven’t been changed. These documents have just been used in the submission to the Institutional Review Board, so they are at hand. Why not file them with the registration and post them on the public face sheet on ClinicalTrials.gov? To do otherwise is to start off with a huge loophole that has been chronically exploited.
-
Results: While the Sponsors [PHARMA] have succeeded in holding on to their claima of keeping the raw data proprietary [secret], they have no such claim on the results. After all, it’s what they themselves publish in the journal articles they generate. The Results Database on ClinicalTrials.gov should have the results of the predefined Primary and Secondary Outcome Parameters clearly labeled. Any other Outcomes should be labeled ‘exploratory’ or ‘non-Protocol’. In addition, since they have already been calculated for submission, there’s no reason to delay posting them. Posting results at the time of submission to the FDA is essential. Why? So Journal editors, peer reviewers, you and me can see them. And the whole point of this enterprise is to deal with the widespread corruption in the reporting of clinical trials in our literature!
It would be the height of comedy to expect anyone to take the word of the PHARMA Sponsors that late-filed Protocol declarations are accurate or that the belated Results filings were the same as those reported to the FDA. The mis-reporting and non-reporting have been too ubiquitous and too destructive for that. So these filings need to be checked and certified, best done by the FDA who is the responsible and accountable agency in this case. It’s time to evoke evidence-based medicine’s real meaning – evidence in the legal sense. Stay tuned…
This caught my eye today and kind of confirms what I’ve always suspected. Amazing it took fifty years to look at this on a large scale. But this appears to be very well done, in terms of sheer numbers of subjects and years of follow up.
http://jamanetwork.com/journals/jamapsychiatry/article-abstract/2552796
Intuition says “yes.” Experience says “yes.” And I’ve had the same hunch and stopped the BCPs every once in a while with improvement in dysphoria most of the time.
It’s always a palate cleanser to find a well-designed study like this.