Pharmalot
By Ed Silverman
November 16, 2015
As a US Senate committee meets on Tuesday to consider Dr. Robert Califf for the top job at the Food and Drug Administration, an open question remains whether he is biased toward industry. Califf is held in high regard by drug makers and academics alike. A cardiologist by training, he spent years as a professor at the Duke University School of Medicine and is one of the most influential biomedical authors in the world. He’s also run numerous clinical trials and served on various FDA advisory committees. But ever since Califf was named a deputy commissioner at the FDA in January and then nominated to be chief regulator in September, his long-standing working relationship with the pharmaceutical industry has prompted debate. Among those opposing his nomination are Democratic presidential aspirant Bernie Sanders and Public Citizen, a consumer advocacy group.
Califf was the founding director of the Duke Clinical Research Institute, which conducts studies for companies. Last year, six drug makers — including Merck and Novartis — partly supported his salary, and several others paid him for consulting work. He has also authored numerous papers with industry researchers. No other commissioner in the recent past has held such close ties to pharmaceutical manufacturers. [The last commissioner, Margaret Hamburg, for example, was a career public health administrator.] All this has led some to challenge whether Califf can be an honest broker. “I do think hard questions should be asked,” said Daniel Carpenter, a Harvard University political scientist, who has studied the FDA but has not taken a position on Califf. “He will have a fair amount of power to push for change”…
Ed continues with a good summary of Dr. Califf’s assets and debits if you’re not already familiar with them. See also:
Boston Globe
By Sheila Kaplan
November 17, 2015
Unfortunately, this kind of information only raises questions, but doesn’t answer them. And that’s likely to be true of the hearings. Of course he will answer the congressional queries and say the right things. And he’s obviously accomplished as a scientist and as an administrator, so I would be very surprised if he’s not confirmed. I just searched this blog for
Califf looking to review what I said earlier, and it popped up an unexpected link from two years ago – before my more recent comments [
at least it’s movement…]. Dr. Califf was the senior author on a non-inferiority study between the new anticoagulant [blood-thinner],
Xarelto®, and the old stand-by,
Warfarin. I was writing about it for other reasons. But on a lark, I looked at the acknowledgements in the paper:
Supported by Johnson & Johnson Pharmaceutical Research and Development and Bayer HealthCare…
Dr. Califf, receiving consulting fees from Kowa, Nile, Orexigen, Sanofi Aventis, Novartis, and Xoma and grant support from Novartis, Merck, and Amilyn/Lilly and having an equity interest in Nitrox…
No kickback from Johnson & Johnson or Bayer? Well that’s kind of encouraging, though every other author was tainted by a Johnson & Johnson or Bayer connection, some being employees. But it got me thinking about some things. Xarelto® isn’t better than Warfarin, it’s just more convenient [by the way, Xarelto® costs a mint]. You don’t have to get frequent blood tests [a nuisance]. But if you’re in a car wreck and bleeding to death, Vitamin K will reverse the effect of Warfarin. There’s no antidote for Xarelto®. So predictably, the suits are beginning to appear about deaths from fatal bleeding in car crashes with Xarelto®. Looking at that paper, a 2+ year comparison of bleeding events in a large cohort, Xarelto® actually came out a little better than Warfarin. And there were fatal bleeding events with both. So Dr. Califf was a participant in an industry-funded Clinical Trial obviously aimed at selling a profitable new drug. As best I could tell, the trial was properly conducted with straightforward conclusions. I believed the study.
I would prefer that Dr. Califf were as clean as a whistle and had no financial connections with any pharmaceutical company. And I would be much happier if he didn’t bother messing with drugs of convenience. Even reading his paper, I wouldn’t take or recommend Xarelto®. My focus would be on being either being the patient or the doctor in the situation when there was a bad car wreck, and I’d say to hell with convenience if I could save a life with a Vitamin shot instead of watching someone bleed to death, I don’t mind insisting on the countless routine blood tests it took to keep that possibility open. But that’s my clinical decision, that would be made in concert with my patient when we’re both playing with a fully informed deck of cards – including this study. I said that to my friend [which was the whole point of my even looking at the paper], and he chose Xarelto® anyway. That’s what his doctor recommended, and that’s what he did [by getting a needed scholarship from J&J]. Their choice. But my friend was fully informed, so I did my job. And in so far as I could tell, so did Dr. Califf [I hope. See this article by the POGO Editor in Chief: Drug Problems: Nominee to Head FDA Led Clinical Trial FDA Faulted].
Looking at other things I’ve written about Dr. Califf, the one real negative was guilt by association [predictable repetitions…]. It was an editorial in the New England Journal of Medicine, Revisiting the Commercial–Academic Interface, by Dr. Jeffrey Drazen supporting Dr. Califf for FDA head [see also a contrarian frame of mind…, wtf?…, wtf? for real…, a narrative…, not so proud…]. In the first place, since when is the editorship of the NEJM a position from which to weigh in on such matters? And Dr. Drazen’s recent showing with his editorial and series on industry-tainted authors writing review articles was an outrageous rationalization, worthy of considering a change in his employment. He’s the paradigm of an industry-friendly guy who assured us fifteen years ago that his PHARMA-connections were behind him and it was… well, it just wasn’t true [sleeper cell comes to mind]. I have no idea how he and Dr. Califf are connected, but I don’t have a bit of question about Dr. Drazen’s Conflicts of Interest. So I see that endorsement from Dr. Drazen as a major black-mark on Dr. Califf’s resume.
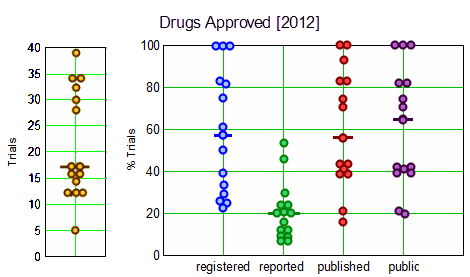
But when it’s all said and done, I don’t really think that the FDA is a major problem except in two areas. I don’t like the fact that the boss over-rode the opinion of the evaluator on drugs like Zoloft or Latuda, but those aren’t my main beefs. They are that the FDA continues to honor industry’s claim that Clinical Trial data and the submitted Clinical Study Reports or Individualized Participant Data are proprietary – keeping secrets for industry – and have made none of the moves towards data transparency like those occurring in the European Medicines Agency. My second complaint is that the trials known as Phase IV [post-marketing] Trials which should be rigorous and ongoing are essentially worthless. They’re done by PHARMA, and rarely mentioned. In reading about a drug, even late in its patent-life, we still mostly hear about those PHASE III Clinical Trials done to get the various approvals. We need to know how the drugs perform [or don’t perform] once they’re out there, and we don’t know – at least in any formal way. The whole Contract Research Organization industry was built to get drugs approved. Once approved, they’re in the hands of the marketeers and then later the Civil and Federal Courts. The latter may be getting better at punishment, but that’s hardly the point.
What matters is what happens once the drug is in general use. I can "vet" products I order on-line from Amazon.com not long after they appear [Amazon.com is the major store for those of us who line in the woods], but even as a doctor, I can’t do that with drugs until they’re almost out of patent except by word of mouth or trial and error. Dr. Healy’s giving it a really serious try with RxISK, but he’s going to need a lot of help. The FDA has a reporting system but it’s woefully lackluster.
The FDA isn’t mandated to do anything but certify safety and minimal efficacy. The agency has no control over drug pricing, and really isn’t set up to survey advertising in a comprehensive way. It just picks up on big lies, not spin. So the questions that matter in today’s hearings are about
 Data Transparency
Data Transparency and
post-marketing Trials. I doubt they’ll be asked, but I sure hope they are. And when it comes down to it, the real question is whether Dr. Califf is a
person of integrity who will make the right hard decisions when the issues that really matter come across his desk [two of my favorite unlikely heros,
James Comey and
Earl Warren, come to mind]? Or will he follow Dr. Drazen’s
sleeper cell mentality? Will the Office make the man? I don’t know how one knows something like that in advance…
post-hearing update:
Boston Globe
Sheila Kaplan
November 17, 2015
Regulatory Affairs
By Zachary Brennan
17 November 2015
Washington Post
By Brady Dennis
November 17, 2015


 But my surprise was that the AMA voted to oppose these ads. That says something positive about our medical community that was unexpected. Who knows? Maybe next on the agenda will be a move to stop ordering un-necessary scans and lab tests? to actively oppose the gajillion fee-churning maneuvers that happen in our emergency rooms and hospitals [including inappropriate screening]. And how about those faux-televisions in the waiting rooms with their infinitely looping infomercials for diabetic supplies and vitamins? Doctor-Power. I like it!
But my surprise was that the AMA voted to oppose these ads. That says something positive about our medical community that was unexpected. Who knows? Maybe next on the agenda will be a move to stop ordering un-necessary scans and lab tests? to actively oppose the gajillion fee-churning maneuvers that happen in our emergency rooms and hospitals [including inappropriate screening]. And how about those faux-televisions in the waiting rooms with their infinitely looping infomercials for diabetic supplies and vitamins? Doctor-Power. I like it! Data Transparency and post-marketing Trials. I doubt they’ll be asked, but I sure hope they are. And when it comes down to it, the real question is whether Dr. Califf is a person of integrity who will make the right hard decisions when the issues that really matter come across his desk [two of my favorite unlikely heros,
Data Transparency and post-marketing Trials. I doubt they’ll be asked, but I sure hope they are. And when it comes down to it, the real question is whether Dr. Califf is a person of integrity who will make the right hard decisions when the issues that really matter come across his desk [two of my favorite unlikely heros, 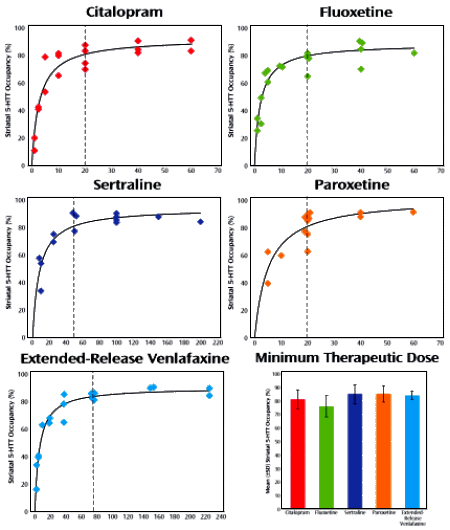
 leg [superhighway], and our top-speed-low-sixty-miles-per-hour Morrris was definitely out classed, but we made it to the outskirts of Paris at midnight. The next morning, we decided that a ride down the Champs-Élysées would be a nice way to end our European adventures before getting on the Ferry, and soon onto the plane home.
leg [superhighway], and our top-speed-low-sixty-miles-per-hour Morrris was definitely out classed, but we made it to the outskirts of Paris at midnight. The next morning, we decided that a ride down the Champs-Élysées would be a nice way to end our European adventures before getting on the Ferry, and soon onto the plane home.