The original study’s authors say the study shouldn’t be retracted because it was conducted properly. “Retraction is typically fraud or you completely screwed something up and it’s not right,” Dr. Ryan says. In the original Study 329, Dr. Ryan says the paper accurately showed that paroxetine didn’t significantly improve depression on the primary outcome measure, a questionnaire that assessed depression, compared with a placebo. But other measures of depression in the study suggested that paroxetine benefited the participants, he says. The analysis and reporting of side effects in the publication was “entirely comparable” to how side effects in other antidepressant studies were being analyzed and reported at the time, Dr. Ryan says.
Our paper was about setting the record straight and data transparency. Both Study 329 authors [Keller and Ryan] could’ve helped with those things instead of adopting their monotonous defensive and dismissive posture. Same for JAACAP Editor Andres Martin and AACAP President Joshi. But their responses were actually expected. It wasn’t enough for a touch of paralysis. Maybe it was Steven Brill’s series in the Huffington Post [America’s Most Admired Lawbreaker] about J&J’s misadventures with Risperdal, an epic I had spent a lot of time on including going to one of the nuclear trials. I was disappointed that the series got so little mention. It’s a particularly egregious example of commercial interests massively over-riding the medical facts and profiteering on a captive audience of the elderly, the institutionalized, and impaired youth. Both Paxil Study 329 and the J&J Risperdal narrative are in the past, so we all already know about them and perhaps are just tired – scandal fatigue. But then I looked back at the posts I’d started and abandoned and it was obvious why I had my touch of paralysis. There was something I wanted to say, but I didn’t have an airtight case. There’s plausible deniability all around that I can’t counter. So I thought instead of going back and forth, I’d just say it and move on.
|
Ketamine is an anesthetic, sold on the streets as the club drug Special K, and is much mentioned as an antidepressant for Treatment Resistant Depression. It began to be discussed around the time the CNS drug pipeline from PHARMA ran dry – a flurry among those who had shepherded the psychopharmacology waves of the twenty-five years following the introduction of Prozac®. Ketamine is given intravenously and its beneficial effect is short-lived [a week +]. I assumed it was a Hail Mary Pass – a long shot by people who had made a career out of discussing new biomedical psychiatric treatments just around the corner who suddenly didn’t have anything new to talk about. The notion of an I.V. anesthetic/street-drug with a short duration of action being a viable therapy just didn’t fit my idea of psychiatric practice. I’ve avoided discussing Ketamine here because my cynicism is based more on the climate and my opinion of the people who have jumped on the band-wagon than anything particularly scientific or medical. There’s more than enough bias going around. So why add to the din? But now there are two recent meta-analysis/review articles about Ketamine and other glutamate receptor modulators that made me change my mind. First, the Cochrane Systematic Review:
PLAIN LANGUAGE SUMMARY
The Cochrane Library 2015, Issue 9.
What does the evidence from the review tell us?
Among the 11 drugs included in this review, only ketamine was more effective than placebo at reducing symptoms of depression. These effects lasted no more than one week after treatment and clearly disappeared after two weeks. Ketamine did, however, cause more confusion and emotional blunting than placebo. These findings were based on low quality evidence.
My summary of the review: Ketamine has an ‘antidepressant’ effect for a week or so. There’s no way to blind the studies because of the up front club drug effect, so there’s no solid way to differentiate Ketamine’s feel-good versus its specific antidepressant properties.
The Cochrane Systematic Reviews are as close as we can come with the current level of data transparency to having reports on various medical treatments that are comprehensive and free of outside influence. While nothing’s perfect, the rigor and techniques used to produce these reports is certainly impressive. They are published in brief or in the encyclopedic variety. I included the PLAIN LANGUAGE SUMMARY and linked the abstract. The fullest report is a 326 page document. In this case, the reviewers rated the quality of the data as pretty low.
On the other side of the coin, this month’s AJP has another review/meta-analysis of the Ketamine studies done by Dr. Jeffrey Newport [University of Miami] and the members of an APA Research Council [I can’t locate the
Research Task Force on Novel Biomarkers and Treatments, but I did find the
APA Council on Research – members in red]:
by D. Jeffrey Newport, Linda L. Carpenter, William M. McDonald, James B. Potash, Mauricio Tohen, and Charles B. Nemeroff, The APA Council of Research Task Force on Novel Biomarkers and Treatments
American Journal of Psychiatry. 2015 172:950–966.
… Other NMDA antagonists failed to consistently demonstrate efficacy; however, two partial agonists at the NMDA coagonist site, D-cycloserine and rapastinel, significantly reduced depressive symptoms without psychotomimetic or dissociative effects.
Conclusions: The antidepressant efficacy of ketamine, and perhaps D-cycloserine and rapastinel, holds promise for future glutamate-modulating strategies; however, the ineffectiveness of other NMDA antagonists suggests that any forthcoming advances will depend on improving our understanding of ketamine’s mechanism of action. The fleeting nature of ketamine’s therapeutic benefit, coupled with its potential for abuse and neurotoxicity, suggest that its use in the clinical setting warrants caution.
My summary of the review: Ketamine has an ‘antidepressant’ effect for a week or so. The authors are sure taken with rapastinel [GLYX-13] in spite of the fact there’s only really one study – a phase 2 trial with only company authors.
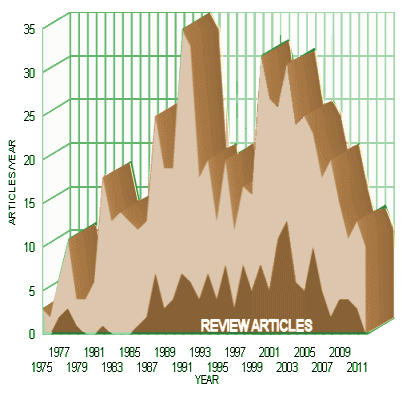 I was recently describing NIMH chief Tom Insel as a breakthrough·freak… – always focusing on some future leap forward in biomedical psychiatric treatment. He is certainly not alone in that category. Before his fall from grace, the senior author in this review lead the pack of the breakthrough·freaks – Dr. Charles Nemeroff [once nick-named "bling-bling" because of his penchant for new novel treatment possibities]. His downfall came with review articles of treatments where he was exposed as having undeclared financial interests – see hubris… and sally’s world…]. I once even compiled his review article publication rate.
I was recently describing NIMH chief Tom Insel as a breakthrough·freak… – always focusing on some future leap forward in biomedical psychiatric treatment. He is certainly not alone in that category. Before his fall from grace, the senior author in this review lead the pack of the breakthrough·freaks – Dr. Charles Nemeroff [once nick-named "bling-bling" because of his penchant for new novel treatment possibities]. His downfall came with review articles of treatments where he was exposed as having undeclared financial interests – see hubris… and sally’s world…]. I once even compiled his review article publication rate.
Given Dr. Nemeroff’s history of allowing commercial interests to slip into review articles, one would be remiss in not looking carefully at an article like this review of Ketamine. And it’s another breakthrough kind of treatment like his recent interests in personalized medicine [genetic and other testing to pick medications, also outrunning its science – Genetic tests for psychiatric drugs spur hope, doubts]. In fact, everyone on the author byline of this AJP article is in the breakthrough freak category [Transcranial Neuromodulation, Vagal Nerve Stimulation, Deep Brain Stimulation, etc] and there are plenty of industry connections in the declaration section of this article, some raising eyebrows.
For the moment, I just want to mention that the Cochrane Meta-analysis, while confirming the short-term effect of Ketamine, found little promise from other glutamate receptor modulators. There’s no commercial breakthrough with Ketamine itself as it’s off patent and readily available. But finding other glutamate receptor modulators that could be developed commercially is another matter altogether. The AJP article mentions them repeatedly and paves the way for their future development – one in particular.
Does the AJP article have "spin"? Of course it does. GLYX-13 apparently comes without the up front "club drug" effect, but, at least according to the
lone study available, it does have an antidepressant effect like Ketamine, and it’s not under patent. Is there anything wrong with a drug company exploring this potential "antidepressant"? No. So what’s my beef? This is a Review Article in the Journal of the
American Psychiatric Association. It’s written by one person plus
The APA Council of Research Task Force on Novel Biomarkers and Treatments [which I’ve not heard of but don’t question exists].
It has the most famously industry-tainted psychiatrist in the world as its senior author. And it has this as its Conflict of Interest statement:
Dr.Newport has received research support from Eli Lilly, GlaxoSmithKline, Janssen, NARSAD, NIH, and Wyeth; he has served on speakers’ bureaus for AstraZeneca, Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth; and he has served on the advisory board for GlaxoSmithKline. Dr. Carpenter has served as a consultant for AbbVie, Magstim, Naurex, Taisho [Helicon], and Takeda/Lundbeck; and she has received research support from Cervel Neurotech, NeoSync, Neuronetics, and NIH. Dr. McDonald has received research support from Cervel Neurotherapeutics, the Health Resources and Services Administration, NIMH, the National Institute of Neurological Disease and Stroke, Neuronetics, Soterix, and the Stanley Foundation; he has served as a consultant for the Center for Devices and Radiological Health, the Food and Drug Administration, and the Neurological Devices Panel of the Medical Devices Advisory Committee; he is a member of the APA Council on Research and Quality representing ECT and Neuromodulation Therapies; he holds a contractwith Oxford University Press to co-edit a book on the Clinical Guide to TranscranialMagnetic Stimulation in the Treatment of Depression; he serves on the editorial boards of the American Journal of Geriatric Psychiatry and the Journal of ECT; and he is Section Editor for Current Psychiatry Reports. Dr. Tohen has been employed with Eli Lilly; he has received honoraria from or consulted for Abbott, Alkermes, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Elan, Eli Lilly, Forest, Geodon Richter Plc., Janssen/Johnson and Johnson, Lundbeck, Merck, Pamlab, Otsuka, Roche, Sepracor Wyeth, Sunovion, Teva, and Wiley Publishing; and his spouse has been employed with Eli Lilly. Dr. Nemeroff has received research/grant support from NIH; he has served as a consultant for Allergan, Clintara LLC, Eli Lilly, Gerson Lehrman Group Healthcare and Biomedical Council, Lundbeck, Mitsubishi Tanabe Pharma Development America, Prismic Pharmaceuticals, Roche, Shire, SK Pharma, Taisho Pharmaceutical, Takeda, Total Pain Solutions, and Xhale; he is a shareholder with Abbvie, Celgene, OPKO Health, Seattle Genetics, Titan Pharmaceuticals, and Xhale; he has served on scientific advisory boards for American Foundation for Suicide Prevention, Anxiety Disorders Association of America, Brain and Behavior Research Foundation [formerly NARSAD], Clintara LLC, RiverMend Health LLC, Skyland Trail, and Xhale; he holds patents forMethod and devices for transdermal delivery of lithium [U.S. 6,375,990B1] and Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay [U.S. 7,148,027B2]; he serves on the Board of Directors of American Foundation for Suicide Prevention, Anxiety Disorders Association of America, and Gratitude America; and he has received income sources or equity of $10,000 or more from American Psychiatric Association Publishing, Clintara, CME Outfitters, Takeda, and Xhale. Dr. Potash reports no financial relationships with commercial interests.
And this is what they have to say about Rapastinel [GLYX-13]:
Rapastinel [GLYX-13] Study Like D-cycloserine, rapastinel is a partial agonist at NMDA receptor glycine binding sites. Whether rapastinel, like D-cycloserine, possesses pharmacodynamic specificity that varies by the NMDA receptor subunit on which the glycine binding site resides is unknown. The literature search identified one randomized clinical trial of rapastinel, evaluating the antidepressant efficacy of a single intravenous infusion at four doses [1, 5, 10, and 30 mg/kg] with treatment effects assessed at 2, 4, 8, and 12 hours and 1, 3, 7, and 14 days postinfusion.
Efficacy. No statistically significant differences were observed in rates of treatment response or symptom remission associated with placebo [64% and 42%, respectively] versus rapastinel at any dose [up to 70% and 53%, respectively]. However, statistically significant differences in the reduction of the 17-item HAM-D scores were observed for the 5-mg/kg dose at all intervals except day 14 [peak 17-item HAM-D reduction 3.1 points greater than placebo] and the 10-mg/kg dose at day 1 and day 3 [peak 17-item HAM-D reduction 4.3 points greater than placebo]. Neither the low [1 mg/kg] nor high [30 mg/kg] rapastinel doses were associated with significant greater 17-item HAM-D score reduction than placebo, leading the authors to posit an inverted U-shape dose response curve.
Psychotomimetic and dissociative side effects.The BPRS positive symptom subscale was administered in this trial with no differences observed between the placebo group and any of the four rapastinel treatment groups. The Clinician-Administered Dissociative States Scale was not administered in this study.
Who makes
Rapastinel [GLYX-13]? A company called
Naurex [See Dr. Carpenter’s COI declarations]. Or at least it used to make it. This AJP Review was accepted for publication on
June 29, 2015, and then on July 26, 2015, we read this press release:
DUBLIN and EVANSTON, Ill., July 26, 2015 /PRNewswire/ — Allergan plc [NYSE: AGN], a leading global pharmaceutical company, and Naurex Inc., a clinical-stage biopharmaceutical company developing transformative therapies for challenging disorders of the central nervous system, today announced that they have entered into a definitive agreement under which Allergan will acquire Naurex in an all-cash transaction. Under the terms of the agreement, Allergan will acquire Naurex for a $560 million upfront payment net of cash acquired, $460 million of which is payable upon the closing of the acquisition and $100 million of which is payable by January of 2016 [or upon the closing if the closing has not occurred by such time], as well as potential R&D success-based and sales-threshold milestone payments. The Company remains committed to de-leveraging to below 3.5x debt-to-EBITDA by the end of the first quarter of 2016…
So
now take a look at Dr. Nemeroff’s COI Statement. It is made all the more poignant in that a year before in a
talk about Ketamine he mentions GLYX-13. The part that is of interest is from 5:40 to 6:40. He describes GLYX-13 made by Naurex and then inserts out of the blue, "
of which I, incidentally, have no relationship" [6:18 – 6:21]. Which was apparently sort of true at the time, but I find it impossible to believe that the buyout by a company he did advise was something he didn’t know about. And here’s what comes next:
FierceBiotech
By Damian Garde
September 16, 2015
The brains behind Naurex, a drug developer acquired by Allergan ($AGN) for $560 million up front, have put the band back together, starting a new biotech with their old company’s technology and setting their sights on central nervous system disorders.
The new operation, Aptinyx, is spinning out of Naurex with much of its management team in tow, reuniting founders with the discovery platform behind the depression drug that first caught Allergan’s eye. Aptinyx is getting started with some early-stage oral treatments that modulate the brain’s NMDA receptors, a pathway the company believes has wide potential beyond GLYX-13, Allergan’s new late-stage antidepressant.
Under the terms of its spinout, Aptinyx is collaborating with Allergan on the discovery and preclinical development of some NMDA modulators for undisclosed psychiatric and neurologic disorders, and the Big Pharma has the right to in-license those as they progress. But the new company retains full rights to everything outside that umbrella, and Aptinyx is working through early-stage studies in traumatic brain injury, neuropathic pain, post-traumatic stress disorder and epilepsy.
The arrangement, Aptinyx CEO Norbert Riedel said, makes use of each party’s strengths. Allergan, a multinational giant, is well suited to take GLYX-13 through Phase III development, regulatory review and commercialization. But the company, under CEO Brent Saunders, has made no secret of its aversion to early drug development, preferring to leave that work to biotech and academia in favor of stepping in after the proof-of-concept stage. And Aptinyx is happy to fill that role in NMDA modulation, Riedel said.
As for the company’s proprietary assets, Aptinyx is in the preclinical stage with a handful of assets and expects to identify its first disease targets in the coming months. If everything goes according to plan, the company should be ready to start its first clinical trial in summer 2016, Riedel said.
Aptinyx rallied some of Naurex’s investors to raise seed money for its preclinical projects, but the biotech plans to put together a Series A financing round in the coming months to fund its clinical aspirations, he said.
Riedel, who served as CEO of Naurex, said his old company negotiated what would become Aptinyx into the Allergan buyout deal "to maintain the truly enormous value that remained in the early stage pipeline and our proprietary platform."
|
So back to my touch of paralysis. Review articles are meant to keep doctors up to date on medical information. Reading this one, you would leave it with the idea that there’s some new treatment up ahead coming for people who are depressed but don’t respond to the current drugs. That’s tainted information, in a major review article, in the major psychiatric journal, certified by a group affiliated with the American Psychiatric Association. Everything in the chain is "legal," and I look like some kind of lunatic muckraker for even bringing it up. Yet in a rational world, there should never be a review article with that kind of COI statement. The major psychiatric journal should never publish a review article by someone with Dr. Nemeroff’s record of using review articles for commercial promotion. And whereas this journal comes to the mailbox of a large number of practicing psychiatrists, one has to work at having access to the more rigorous and reliable Cochrane Systematic Reviews. Every person on this author by-line is an Academic Psychiatrist on a major medical school faculty.
Although no crime has been committed, and under the current state of play, nothing in this chain of events breaks any specific rule, nonetheless, the unacknowledged fingers of commercial enterprise are all over this review/meta-analysis in the American Journal of Psychiatry – the overall purpose of an academic journal has been subverted in plain view. It may seem a small thing, just a bright note about a coming possibility of a new avenue of treatment. But by now, we ought to know better. We ought to know that it’s just this kind of "bright note" that has edged mediocre and sometimes dangerous drugs into blockbusters leading to overdiagnosis and overmedication. Conflicts of Interest, whether declared or not, are by definition a central enemy in the free flow of honest medical information. In many areas of medicine, that information conduit has been captured and turned into an advertising platform. And Psychiatry has, unfortunately, lead the field…
 And as for my touch of paralysis, when I read the review in the AJP and chased down the COI and GLYX-13 connections to big doings on the industry side of drug development, the voices inside and outside of my head said that what I found was only circumstantial evidence – wouldn’t hold up in court. I wanted to write about it, but knew through anyone else’s eyes, it could be discounted as idle speculation by someone who has become paranoid by seeing too much corruption, and now is seeing it in everything. But on the other side, too many of us, myself included, sat quietly through decades of this kind of subtle industry influence and look where it got us. We settled for the standards used in the criminal justice system. The real standard in medicine and science in general is that there should not be the appearance of bias, not that there needed to be enough evidence to take to a court of law. And we all should know that declaring conflicts of interest is not the same as not having conflicts of interest. So in the end, I decided that in this case, silence isn’t golden. I’d rather be wrong and say I’m sorry later than be right and have kept my mouth shut like so many to the detriment of our specialty and our patients. I guess I’m saying that this really still is the elephant in the room that needs to be talked about… And as for my touch of paralysis, when I read the review in the AJP and chased down the COI and GLYX-13 connections to big doings on the industry side of drug development, the voices inside and outside of my head said that what I found was only circumstantial evidence – wouldn’t hold up in court. I wanted to write about it, but knew through anyone else’s eyes, it could be discounted as idle speculation by someone who has become paranoid by seeing too much corruption, and now is seeing it in everything. But on the other side, too many of us, myself included, sat quietly through decades of this kind of subtle industry influence and look where it got us. We settled for the standards used in the criminal justice system. The real standard in medicine and science in general is that there should not be the appearance of bias, not that there needed to be enough evidence to take to a court of law. And we all should know that declaring conflicts of interest is not the same as not having conflicts of interest. So in the end, I decided that in this case, silence isn’t golden. I’d rather be wrong and say I’m sorry later than be right and have kept my mouth shut like so many to the detriment of our specialty and our patients. I guess I’m saying that this really still is the elephant in the room that needs to be talked about…
|
 My early years were spent living on the campus of a boys military school where my father taught and coached, a school I later attended for a few years. It was founded and run by a family of [fundamentalist] Presbyterians [of the Scottish John Calvin kind] – an overdose of the Protestant Work Ethic, a strict unforgiving morality, and chapel at least twice a day. By their read, the appearance of sin was almost as bad as the real thing. In mid high school, I fled to a normal 1950s school where I thrived – free at last! But I think some of that Calvinism must’ve gone all epigenetic in those years, because I still can’t think of any reason for physicians to be paid by drug companies unless they work for them. And that feels like it’s in my DNA. Given the choice, I’d rather not have to deal with COIs at all. So although I’m glad that articles now have industry affiliations listed, that there’s a ProPublic Database, and that we passed a Sunshine Act, in my heart of hearts, I’m a Calvinist about doctors with industry ties – the appearance is almost as bad as the real thing. And there’s nothing in all the corruption and scandal [particularly in my specialty of psychiatry] to temper my uncharacteristic moral rigidity on this point. I now read articles in this specific order:
My early years were spent living on the campus of a boys military school where my father taught and coached, a school I later attended for a few years. It was founded and run by a family of [fundamentalist] Presbyterians [of the Scottish John Calvin kind] – an overdose of the Protestant Work Ethic, a strict unforgiving morality, and chapel at least twice a day. By their read, the appearance of sin was almost as bad as the real thing. In mid high school, I fled to a normal 1950s school where I thrived – free at last! But I think some of that Calvinism must’ve gone all epigenetic in those years, because I still can’t think of any reason for physicians to be paid by drug companies unless they work for them. And that feels like it’s in my DNA. Given the choice, I’d rather not have to deal with COIs at all. So although I’m glad that articles now have industry affiliations listed, that there’s a ProPublic Database, and that we passed a Sunshine Act, in my heart of hearts, I’m a Calvinist about doctors with industry ties – the appearance is almost as bad as the real thing. And there’s nothing in all the corruption and scandal [particularly in my specialty of psychiatry] to temper my uncharacteristic moral rigidity on this point. I now read articles in this specific order:

 Given the NIMH output during the twenty years under discussion, there’s little evidence that exerting this much control has been very productive – too much form without accompanying substance. And it doesn’t take much reading of the strategic directives above to see that there is a theme to what is considered strategic [we might call it Insel’s theme]. Hopefully, the next Director will have more faith in our research community. Likewise, given the freedom to follow their intuition, they will likely be more committed [and more productive]…
Given the NIMH output during the twenty years under discussion, there’s little evidence that exerting this much control has been very productive – too much form without accompanying substance. And it doesn’t take much reading of the strategic directives above to see that there is a theme to what is considered strategic [we might call it Insel’s theme]. Hopefully, the next Director will have more faith in our research community. Likewise, given the freedom to follow their intuition, they will likely be more committed [and more productive]… 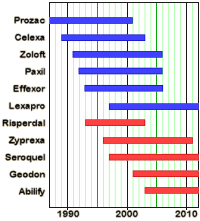 Tom Insel came to the NIMH at the height of the rise of neuroscience. It was a new century. The future DSM-5 team was just setting its sights on finally adding the hard science fixings to the psychiatrized brain. The drugs were steadily pouring from the pharmaceutical pipeline and were hitting the top of the charts in sales. New drug strategies were closing in on the treatment resistant cases. With the human genome cracked and a maturing of neuroimaging, it was just a matter of time before the dream of the new psychiatry would be a reality. A few years into his reign, Dr. Insel announced this new paradigm, Psychiatry was to become
Tom Insel came to the NIMH at the height of the rise of neuroscience. It was a new century. The future DSM-5 team was just setting its sights on finally adding the hard science fixings to the psychiatrized brain. The drugs were steadily pouring from the pharmaceutical pipeline and were hitting the top of the charts in sales. New drug strategies were closing in on the treatment resistant cases. With the human genome cracked and a maturing of neuroimaging, it was just a matter of time before the dream of the new psychiatry would be a reality. A few years into his reign, Dr. Insel announced this new paradigm, Psychiatry was to become 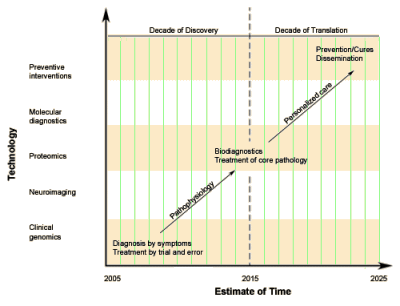
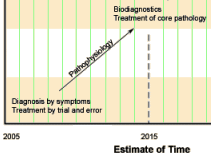 Dr. Insel went straight from his psychiatry residency [1976-1979] to the NIMH [1979-1994], then to the Yerkes Primate Center [1994-1999], followed by administrating a translational behavioral neuroscience program [1999-2002], then he finally returned to the NIMH as Director [2002-2015]. I happen to know this history [which is devoid of any clinical experience] because I was trying to understand how he had come to have such a simplistic view of mental illness. He apparently believes those population estimates he often quotes are accurate ["… by 20xx, depression will be the number x cause of xxxxx"] [epidemic?]. He seems to have accepted the notion that all mental illnesses are biomedical circuit problems and the solution to the mental health system is badly broken is a new crop of drugs/treatments and some kind of outreach to find the untreated cases. Having failed to achieve the 2015 goal of "Biodiagnostics" and "Treatment of Core Pathology", he just went ahead and declared a new Biodiagnostic System anyway – the RDoC [whatever that is].
Dr. Insel went straight from his psychiatry residency [1976-1979] to the NIMH [1979-1994], then to the Yerkes Primate Center [1994-1999], followed by administrating a translational behavioral neuroscience program [1999-2002], then he finally returned to the NIMH as Director [2002-2015]. I happen to know this history [which is devoid of any clinical experience] because I was trying to understand how he had come to have such a simplistic view of mental illness. He apparently believes those population estimates he often quotes are accurate ["… by 20xx, depression will be the number x cause of xxxxx"] [epidemic?]. He seems to have accepted the notion that all mental illnesses are biomedical circuit problems and the solution to the mental health system is badly broken is a new crop of drugs/treatments and some kind of outreach to find the untreated cases. Having failed to achieve the 2015 goal of "Biodiagnostics" and "Treatment of Core Pathology", he just went ahead and declared a new Biodiagnostic System anyway – the RDoC [whatever that is].  A US Senator has asked the Federal Trade Commission to investigate whether drug makers are hiking prices and then restricting distribution to prevent generic drug makers from making lower-cost versions of their medicines. In
A US Senator has asked the Federal Trade Commission to investigate whether drug makers are hiking prices and then restricting distribution to prevent generic drug makers from making lower-cost versions of their medicines. In  As we explained earlier this week
As we explained earlier this week President Obama’s nominee to lead the Food and Drug Administration recently coauthored a series of scientific papers raising concerns about the agency’s oversight of clinical trials but asked that his name be removed before publication, according to other authors. Dr. Robert Califf, the Duke cardiologist first named a deputy commissioner in January and then nominated for the top post last month, was the driving force behind the series, which was published in the October issue of the journal Clinical Trials.
President Obama’s nominee to lead the Food and Drug Administration recently coauthored a series of scientific papers raising concerns about the agency’s oversight of clinical trials but asked that his name be removed before publication, according to other authors. Dr. Robert Califf, the Duke cardiologist first named a deputy commissioner in January and then nominated for the top post last month, was the driving force behind the series, which was published in the October issue of the journal Clinical Trials.
 I was recently describing NIMH chief Tom Insel as
I was recently describing NIMH chief Tom Insel as  And as for my touch of paralysis, when I read the review in the AJP and chased down the COI and GLYX-13 connections to big doings on the industry side of drug development, the voices inside and outside of my head said that what I found was only circumstantial evidence – wouldn’t hold up in court. I wanted to write about it, but knew through anyone else’s eyes, it could be discounted as idle speculation by someone who has become paranoid by seeing too much corruption, and now is seeing it in everything. But on the other side, too many of us, myself included, sat quietly through decades of this kind of subtle industry influence and look where it got us. We settled for the standards used in the criminal justice system. The real standard in medicine and science in general is that there should not be the appearance of bias, not that there needed to be enough evidence to take to a court of law. And we all should know that declaring conflicts of interest is not the same as not having conflicts of interest. So in the end, I decided that in this case, silence isn’t golden. I’d rather be wrong and say I’m sorry later than be right and have kept my mouth shut like so many to the detriment of our specialty and our patients. I guess I’m saying that this really still is the elephant in the room that needs to be talked about…
And as for my touch of paralysis, when I read the review in the AJP and chased down the COI and GLYX-13 connections to big doings on the industry side of drug development, the voices inside and outside of my head said that what I found was only circumstantial evidence – wouldn’t hold up in court. I wanted to write about it, but knew through anyone else’s eyes, it could be discounted as idle speculation by someone who has become paranoid by seeing too much corruption, and now is seeing it in everything. But on the other side, too many of us, myself included, sat quietly through decades of this kind of subtle industry influence and look where it got us. We settled for the standards used in the criminal justice system. The real standard in medicine and science in general is that there should not be the appearance of bias, not that there needed to be enough evidence to take to a court of law. And we all should know that declaring conflicts of interest is not the same as not having conflicts of interest. So in the end, I decided that in this case, silence isn’t golden. I’d rather be wrong and say I’m sorry later than be right and have kept my mouth shut like so many to the detriment of our specialty and our patients. I guess I’m saying that this really still is the elephant in the room that needs to be talked about… insurers, with each blaming the other for the pricing controversy. Drug makers say they offer rebates and discounts to insurers that then shift more out-of-pocket costs onto patients, while insurers argue the prices are set too high in the first place.
insurers, with each blaming the other for the pricing controversy. Drug makers say they offer rebates and discounts to insurers that then shift more out-of-pocket costs onto patients, while insurers argue the prices are set too high in the first place.

 Whether you call it a bubble, a monopoly, a captive audience, or just a mess doesn’t much matter. The point is that the problem isn’t Martin Shkreli. He’s just another greedy baby who jumped into the feeding frenzy. There are plenty more where he came from. He doesn’t have the style points of an Alex Gorsky [
Whether you call it a bubble, a monopoly, a captive audience, or just a mess doesn’t much matter. The point is that the problem isn’t Martin Shkreli. He’s just another greedy baby who jumped into the feeding frenzy. There are plenty more where he came from. He doesn’t have the style points of an Alex Gorsky [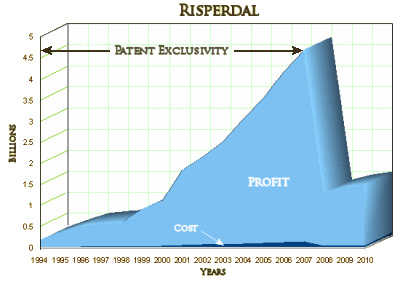 a medical writing firm less than a mile away [Excerta Medica] turning out journal articles faster than they could locate KOL authors to sign up, a keen sense for backroom wheelings and dealings, and winning interpersonal ways, Gorsky set out to create the markets needed to become a blockbuster and then some. The story is masterfully told in the Huffington Post fifteen part series by Steven Brill. It’s all on-line just waiting for you to read it. And you won’t have a bit of trouble understanding why he called it
a medical writing firm less than a mile away [Excerta Medica] turning out journal articles faster than they could locate KOL authors to sign up, a keen sense for backroom wheelings and dealings, and winning interpersonal ways, Gorsky set out to create the markets needed to become a blockbuster and then some. The story is masterfully told in the Huffington Post fifteen part series by Steven Brill. It’s all on-line just waiting for you to read it. And you won’t have a bit of trouble understanding why he called it