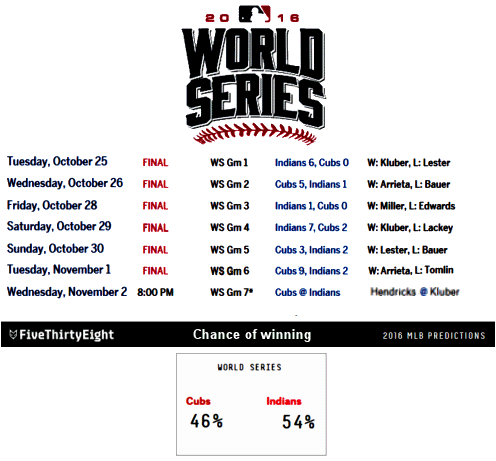
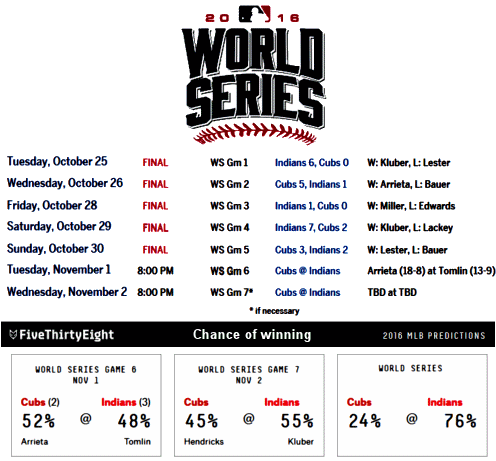
Posted on Monday 14 November 2016
PSYCHIATRICNEWSby Anne Huben-Kearney, R.N., B.S.N., M.P.A.October 31, 2016
The importance of good documentation cannot be overstated. Documentation can provide evidence of care provided or not provided; serve to promote patient safety and minimize error; ensure regulatory, accreditation, and reimbursement compliance; provide an effective defense in a medical malpractice claim, and even prevent a claim from going forward. The medical record, whether in paper or electronic form, is a legal document. The medical record is…
Scrutinized by the plaintiff and defense attorneys.
Documentation includes the notes in the psychiatric medical record itself, including informed consent, telephone messages/responses, prescriptions, and, if used, email communication with the patient.
Documentation should have these characteristics:
Allied World, through its subsidiaries, is a global provider of innovative property, casualty and specialty insurance and reinsurance solutions. Allied World is the APA-endorsed carrier for the professional liability program through its strategic relationship with the American Professional Agency Inc., the Program Administrator. This information is provided as a risk management resource and should not be construed as legal, technical, or clinical advice. Consult your professional advisors or legal counsel for guidance on issues specific to you. This material may not be reproduced without the permission of Allied World. Risk management services are provided by or arranged through AWAC Services Co., a member company of Allied World. Anne Huben-Kearney, R.N., B.S.N., M.P.A., is assistant vice president of the Psychiatric and Healthcare Risk Management Group of AWAC Services Company, a member company of Allied World.
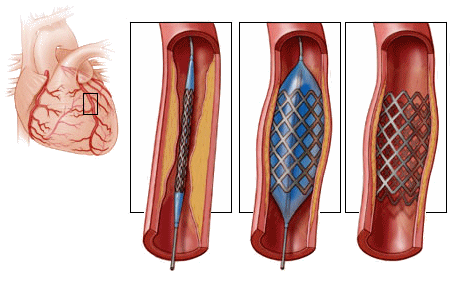 At the time I was an Intern, we were just begining to realize that patients died from arrhythmias in the period just after a heart attack and had begun to monitor the EKG – a major advance. Now there’s a monitor in every hospital room, but in the period just after a heart attack, the patients don’t go to their rooms. They usually go to a cath lab for a stent that opens the clogged artery [often for good].
At the time I was an Intern, we were just begining to realize that patients died from arrhythmias in the period just after a heart attack and had begun to monitor the EKG – a major advance. Now there’s a monitor in every hospital room, but in the period just after a heart attack, the patients don’t go to their rooms. They usually go to a cath lab for a stent that opens the clogged artery [often for good]. But those changes were predictable. Medical science regularly marches upward. The changes I’m talking about are things like this article from the PSYCHIATRICNEWS focusing on Risk Management. Fifty years ago, had you handed me an article about Risk Management, I would’ve been curious to know what it was about – assuming that it would be about the risk patients faced from their diseases, or maybe their treatments. It wouldn’t have ever occurred to me that it would be about managing he doctors’ risk from the patients. In the first paragraph of the article where it gives the rationale for this kind of documentation, it mentions promote patient safety and minimize error in passing, but most of it is about preemptively heading off charges of negligence and/or malpractice. And it ends with Failure to document is not only unethical but can lead to license revocation, restriction, or disciplinary actions and also the inability to defend a malpractice claim – suggesting that one should Provides evidence of professional credibility in every note.
 I suppose the suggestion that this kind of micro-documentation might make sense if the time allotted for a patient visit included the time it takes to write this kind of note, but that’s not the case. And we might add in the use of an Electronic Medical Record [EMR] system built to contain all of this information [most of which don’t have psychiatry in mind]. And perhaps others are more comfortable than I am being a clinician who is looking primarily at a computer screen. But those are just a few examples of the ground clutter choking medical practice. There are plenty of other things – things like screening [BP, P, PHQ-9, etc.].
I suppose the suggestion that this kind of micro-documentation might make sense if the time allotted for a patient visit included the time it takes to write this kind of note, but that’s not the case. And we might add in the use of an Electronic Medical Record [EMR] system built to contain all of this information [most of which don’t have psychiatry in mind]. And perhaps others are more comfortable than I am being a clinician who is looking primarily at a computer screen. But those are just a few examples of the ground clutter choking medical practice. There are plenty of other things – things like screening [BP, P, PHQ-9, etc.].
As a free clinic staffed by retirees with only one paid employee [the Director], our little clinic was a great place to work even though we operated on a wing, a prayer, donated and generic drugs, and old people. Then came the decision to take insurance from those that had it – to get certified. Over the last year, the volunteer physicians, nurses, pharmacists, etc have all left [except for me] – and I’m finally mustering my way out over the next 3 or 4 months. It has been heartbreaking to see things change so quickly in spite of the well-meaning powers that be who tried to keep it from happening. I naively thought I could Ostrich my way through it, but I can’t bring it off in spite of trying.