I bookmarked this back during the holidays because of the parts that are highlighted, but that was back when we thought that the EMA Data Transparency was settled, but awaiting the resolution of the AbbeVie/InterMune suits [see
a deal-breaker?…]. Running across it today, I can see why I flagged it:
Reuters
By Jim Finkle
Dec 17, 2013
The U.S. Food and Drug Administration is under pressure from the pharmaceutical industry and lawmakers to undergo an independent security audit, after hackers broke into a computer system used by healthcare companies to submit information to the agency. Drug companies fear the cyber thieves may have accessed corporate secrets that are on file with the agency, such as data about drug manufacturing, clinical trials, marketing plans and other proprietary information. While some lawmakers charge that the hackers breached the FDA’s gateway, compromising confidential business data, the agency argues that the access was limited.
The breach came to light last month when the FDA sent letters to users of an online system at the Center for Biologics Evaluation and Research. The letters said the breach was detected by the FDA on Oct. 15 and that it resulted in the theft of usernames, phone numbers, email addresses and passwords. The U.S. House of Representatives Energy and Commerce Committee launched an investigation, and last week four senior Republican members of that committee sent a letter to FDA Commissioner Margaret Hamburg asking her to immediately launch a third-party audit that would "assess and ensure the adequacy of FDA’s corrective actions" following the breach
Washington-based pharmaceutical industry trade group PhRMA said on Tuesday that it supported the committee’s request for an independent audit. "It is the legal obligation of the Food and Drug Administration to protect companies’ trade secrets and confidential commercial information," PhRMA Vice President Sascha Haverfield said in a statement. The group’s members include Amgen Inc, Daiichi Sankyo, GlaxoSmithKline, Johnson & Johnson, Merck & Co and Novartis AG. The FDA’s breach notification letter, which was published in pharmaceutical trade publications, referred to the compromised system as an "online submission system" at the Center for Biologics Evaluation and Research. That alarmed drugmakers, which provide the FDA with highly sensitive data – which would be priceless to a competitor – when they submit applications seeking approval for new drugs, biologics and medical devices.
In their letter to the FDA, the Energy and Commerce Committee members charged that the attackers had breached the "FDA’s gateway system," compromising confidential business information along with sensitive data about patients enrolled in clinical trials. FDA spokeswoman Jennifer Rodriguez said that was wrong. "The system that was attacked maintains account information for the Biologic Product Deviation Reporting System, the Electronic Blood Establishment Registration System and the Human Cell and Tissue Establishment Registration System," she said. "This system is not used to submit any applications. It is not the electronic gateway that was breached," she added. She also said that the agency was not aware of any attempts to use stolen information for "criminal or other inappropriate purposes." Rodriguez declined to comment on the requests for an outside audit or say whether the breach had affected more than the 14,000 accounts disclosed to date. Tracy Cooley, a spokeswoman for the Biotechnology Industry Organization, another healthcare industry trade group, said her organization also had concerns about the breach. "We support Congress investigating this situation," she said.
It is "
corporate secrets that are on file with the agency, such as data about drug manufacturing, clinical trials, marketing plans and other proprietary information" and "
It is the legal obligation of the Food and Drug Administration to protect companies’ trade secrets and confidential commercial information" that are the two phrases of note. I spoke recently about how PHARMA settles suits rather than letting them go to a decision and always say that "we admit no wrongdoing" as a way of creating no echo [
with no echo…]. No matter what happens, they speak publicly as if the world is as they say it is. They are aggressive in asserting their rights, and never mention the times they’ve been nailed for gross misbehavior. They’ve avoided publishing well over half their clinical trials, have used ghost-writers extensively, and have ignored the obligations of clinicaltrials.gov, but never openly acknowledge any of those things. The entitlement and arrogance of this article makes my blood boil a bit.
I don’t question that those two statements are likely true – that they’ve succeeded in getting the world to accept or even rule that clinical trial data are "corporate secrets" or that they have the FDA including clinical trial data as "confidential commercial information," but I’m beginning to think that it got those designations originally simply because they said that’s what it was, not because the bodies involved actually thought deeply about the question, or made some ruling. Thus far, the only ruling I know of is in the World Trade Organization agreements [see repeal the proprietary data act…, except where necessary to protect the public…, and a crushing setback…] which contains the phrase "except where necessary to protect the public."
Last week, I posted an article which was even better on second reading. It was admittedly written before EMA’s U Turn, but it remains a comprehensive review of the process and ends with some recommendations [it is a thorough review of the issue and is only 34 pages of legal discussion long]:
by Pamela Andanda
IIC – International Review of Intellectual Property and Competition Law. 2013 44[2]:140-177.
The main strategic approaches that have been identified in this paper are summarized below.
-
Researchers should ensure that they reserve the right to publish clinical research findings expeditiously when they sign clinical trial agreements and TTOs that negotiate licensing agreements with the pharmaceutical industry, on behalf of researchers, should equally be sensitized on the need to protect this right since it is an ethical obligation under paragraph 30 of the Declaration of Helsinki.
-
Regulatory authorities should exercise discretion by drawing a clear distinction between the data itself and the health and safety outcome to which the data lead. This approach should enable the regulatory authorities to exercise the freedom to register competing products based on proven health and safety outcomes if bioequivalence can be established by the applicant. This is acceptable under Article 39.3 of TRIPS.
-
Regulatory authorities should be allowed through domestic legislation to rely on published scientific information to approve competitors’ products. As was noted earlier, TRIPS is not a model law to be copied at the domestic level and due to the contested interpretations of Article 39.3, it would be useful for countries to provide clarity in their legislations, which would enable regulatory authorities more freedom in decision making.
-
Regulatory authorities should adopt the strategy, which EMA has so far used to facilitate access to full clinical research reports for independent meta-analysis for public health benefits. This calls for a shift in the current default position from confidentiality to one of disclosure. The strategy equally requires the enactment of access to information legislation to regulate the disclosure of information and clear exceptions such as those contained in the Japanese AAI.
While no one has been clammoring at the door to my cabin asking for my personal analysis of this situation, I thought I’d just throw it out there in case there are passers-by looking for advice and I’m taking a nap. The pharmaceutical industry is a power source to be reckoned with – individual companies and as a group – with stables of bright in-house and contracted lawyers. They are also represented by one of the more powerful lobby groups in Washington, and elsewhere in the world. I think I would be also justified in saying that looking for a wellspring of touchy-feely humanitarian concern in that camp is looking for love in all the wrong places.
The arguments for making the raw information in clinical trials from before the breaking of the study’s blind available for independent review are unassailable – downright Constitutional – "Life, Liberty, and the Pursuit of Happiness." If that weren’t enough, the betrayal of trust by industry in keeping this information secret is now a matter in the public record – the stuff of legend. There’s no real question about the wisdom of Data Transparency, only legalistic arguments about commerce, trade secrets, or other things much lower down on any rational hierarchy of human values.
So we have the age old clash of wisdom versus power. Given the tenacity of the pharmaceutical industry, their track record of success, and their power, neither negotiation nor seeking legislation seem likely to be productive. I reckon that this looks like a job for the courts, which is the only place wisdom has a fighting chance against that kind of power. I finally located what I think is the definitive legal argument to take to the courts with shared conviction:
by Trudo Lemmens and Candice Telfer
American Journal of Law and Medicine. 2012 38:63-112.
While there is a considerable literature on access to essential medicines and human rights, the topic of access to reliable drug safety and effectiveness information has received little or no attention in human rights discourse. The paper argues that access to information related to clinical drug trials is a fundamental component of the right to health. This approach rejoins the claim of authors who characterize clinical drug trials as public goods. Yet, it offers also a legal and moral basis for the immediate implementation of transparency measures, regardless of more fundamental reform of drug regulation. Framing access to clinical trials data as a component of the right to health offers strong support against the argument that transparency measures may violate international trade obligations related to data secrecy and provides a basis for claiming that states have a duty to implement such measures.
The paper first provides a detailed overview of the historical development of clinical trials and results reporting registries. It then analyzes the arguments invoked against mandatory trial registration and results reporting, zooming in on claims based on data secrecy obligations under TRIPS and TRIPS Plus agreements. The paper explores why registries can generally be justified under public interest exceptions in international trade agreements and how the existence of data exclusivity regimes already provides protection against ‘unfair commercial use’ of clinical trials data.
In the final section of the paper, the implementation of clinical trial and results reporting registries is situated in the context of the right to health. This not only promotes individual empowerment in requesting access to relevant health information, but also suggests that states have a duty to develop reliable and publicly accountable information systems. Such systems should enable independent medical research groups and civil society in general to contribute meaningfully to publicly accountable medical research and health product development.
VII. CONCLUSION
We believe that approaching clinical trials registration and results reporting from the perspective of the right to information as a component of the right to health provides significant advantages. It gives advocacy groups, the research community, national governments, and international organizations not only a strong moral but also a legal foundation to reduce the limits imposed on access to clinical trials data by international trade obligations and related national rules. It should enable advocacy groups and health policymakers to argue for the national implementation of mandatory trial registration and results reporting, and should stimulate international organizations such as the WHO and the PAHO to continue with the development of a coherent international system of research transparency. It should also inspire civil society to develop further tools to ensure the transparency and the reliability of medical research. Civil society and, particularly, independent organizations committed to promoting reliable and accountable evidence-informed healthcare systems play a crucial role in this context. Considering the serious problems associated with the design, conduct, reporting of industry-controlled clinical research, and the limits of regulatory control, immediate steps have to be taken to safeguard the reliability of this crucial component of evidence-informed healthcare decision-making. Clinical trials registration and results reporting, we have argued, are crucial pillars of health information governance. A meaningful realization of the right to health is only possible if healthcare decisions, both at the individual and at the systems level, are built on well-governed and publicly accountable health information systems.
The article is 50 pages long, but it’s not legalese. It’s wisdom, which is what this problem needs. The time for negotiation is drawing to a close..

 Here’s something of a brief summary. In April, when AbbVie dropped its injunction, the EMA issued a press release saying they had agreed to "very limited redactions" in the AbbVie clinical study reports – though there was widespread suspicion about what that meant. The BMJ obtained that agreement through an FOI request that showed them to be more extensive, and when compared to the later proposal of the EMA [post U Turn], there were "striking" similarities between this new proposal and the AbbVie agreement documents. Beate Wieseler, head of drug assessment at the German Institute for Quality and Efficiency in Health Care [IQWiG] and a vocal proponent of transparency noticed these same similarities. It seems that the EMA/AbbVie negotiations were protracted and resulted in some pretty significant retractions. And Wieseler challenges the notion that these retractions were really justified as commercially confidential Information [CCI] – or, for that matter, even necessary. So the consensus that the EMA bowed to AbbVie’s demand, and further radically changed their tune seems to be confirmed [though the EMA denies that is the case]. Now we’re again on hold, waiting to see the fine print.
Here’s something of a brief summary. In April, when AbbVie dropped its injunction, the EMA issued a press release saying they had agreed to "very limited redactions" in the AbbVie clinical study reports – though there was widespread suspicion about what that meant. The BMJ obtained that agreement through an FOI request that showed them to be more extensive, and when compared to the later proposal of the EMA [post U Turn], there were "striking" similarities between this new proposal and the AbbVie agreement documents. Beate Wieseler, head of drug assessment at the German Institute for Quality and Efficiency in Health Care [IQWiG] and a vocal proponent of transparency noticed these same similarities. It seems that the EMA/AbbVie negotiations were protracted and resulted in some pretty significant retractions. And Wieseler challenges the notion that these retractions were really justified as commercially confidential Information [CCI] – or, for that matter, even necessary. So the consensus that the EMA bowed to AbbVie’s demand, and further radically changed their tune seems to be confirmed [though the EMA denies that is the case]. Now we’re again on hold, waiting to see the fine print. 

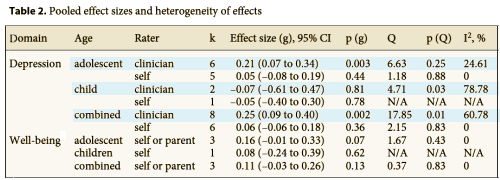


 So I thought I’d go back to the Proprietary Data Act and see why they were given that right in the first place. Only I couldn’t find the Proprietary Data Act anywhere no matter what I put in Google®. That happens, so I wrote everyone I knew asking for leads. They kindly agreed to help, but they couldn’t quite recall – Was it Congress back in 1962? Just not sure. So they looked and couldn’t come up with anything either. So how am I going to mount a campaign to Repeal the Proprietary Data Act if I can’t even find it? I finally heard from some people-in-the-know who had obviously gone on the same Hegira and found nothing either. It just happened apparently. The pharmaceutical companies had the data from the CROs they hired, and they kept it to themselves. There is no Proprietary Data Act. Never was. I guess it’s something like staking a claim in the Klondike during the gold rush or squatter’s rights. So much for my Repeal the Proprietary Data Act Movement.
So I thought I’d go back to the Proprietary Data Act and see why they were given that right in the first place. Only I couldn’t find the Proprietary Data Act anywhere no matter what I put in Google®. That happens, so I wrote everyone I knew asking for leads. They kindly agreed to help, but they couldn’t quite recall – Was it Congress back in 1962? Just not sure. So they looked and couldn’t come up with anything either. So how am I going to mount a campaign to Repeal the Proprietary Data Act if I can’t even find it? I finally heard from some people-in-the-know who had obviously gone on the same Hegira and found nothing either. It just happened apparently. The pharmaceutical companies had the data from the CROs they hired, and they kept it to themselves. There is no Proprietary Data Act. Never was. I guess it’s something like staking a claim in the Klondike during the gold rush or squatter’s rights. So much for my Repeal the Proprietary Data Act Movement. Well, I didn’t know it when I wrote
Well, I didn’t know it when I wrote