NIH Director’s Blog
by Dr. Francis Collins
February 4, 2014
It would seem like there’s never been a better time for drug development. Recent advances in genomics, proteomics, imaging, and other technologies have led to the discovery of more than a thousand risk factors for common diseases—biological changes that ought to hold promise as targets for drugs…
It would appear that the terms genomics, proteomics, and imaging ought to be combined into a single phrase, because we usually hear them together. For that matter, we could throw in recent advances in into a unitary shorthand for recent·advances·in·genomics·proteomics·and·imaging. I thought the phrase was limited to psychiatry/neuroscience, but from the above in appears to be a medicine-wide term to introduce research initiatives that lead us into a brighter future [how the recent·advances·in·genomics·proteomics·and·imaging triad relates specifically to drug development isn’t exactly clear to me, but I don’t want to be too picky].
There are some other stock phrases that come to mind: unmet·clinical·need and global·burden·of·disease. Both of these are used routinely in reports of clinical trials or in pleas for more research funding for some particular project. The latter was almost guaranteed to be in the introduction to the ghost written clinical trial reports that were so popular during the last several decades, the age of psychopharmacology. And unmet·clinical·need is all purpose – can either go in an article to introduce a new drug that’s being hawked as an advance or later when discussing how that last drug wasn’t really very good and we need to find a new one [it’s actually hard to think of a medical situation or grant proposal where you couldn’t throw in an unmet·clinical·need].
And even though it’s only a single word, translation fits into this lexicon because it can be shorthand for so many things. It’s usage overflows its specific meaning these days, but it was intended to mean moving basic science research into something that directly helped patients [it’s also hard to think of a medical situation or grant proposal where you couldn’t throw in a translation metaphor or two]. There’s another newer term that goes in here as well, but I’ll talk about it later – recent·exits·by·companies·from·psychiatry.
Science
by Steven Hyman
March 14, 2014
Last month, the battle against four major diseases received some good news. The U.S. National Institutes of Health [NIH] and 10 of the world’s largest pharmaceutical companies decided that instead of working ineffectively in silos, they would work together to discover therapies for Alzheimer’s disease, type 2 diabetes, rheumatoid arthritis, and lupus. This initiative — the Accelerating Medicines Partnership [AMP] — recognizes that progress toward new therapies for common chronic diseases increasingly requires large-scale collaborative efforts that range from the need to grapple with heterogeneous polygenic disease phenotypes to the validation of biomarkers in large populations. What is disappointing is that, at least for the time being, the consortium dropped schizophrenia from its list, despite vast unmet medical need and substantial, albeit still recent, scientific advances. Was schizophrenia deemed too risky to pursue? If innovative partnerships such as the AMP are not willing to take on common and serious but otherwise neglected disorders such as schizophrenia, then the scientific community will have to find new ways of pooling intellectual and financial resources to address them…
So now we can combine my shorthand with Dr. Hyman’s longhand:
unmet·clinical·need and global·burden·of·disease
Schizophrenia is a severe and disabling brain disorder that also creates enormous costs and challenges for caregivers and for society. Antipsychotic drugs that partially treat hallucinations and delusions were discovered in the early 1950s but have serious side effects and leave entirely untreated schizophrenia’s characteristic cognitive impairments and “negative” symptoms such as blunting of emotion, loss of motivation, and impoverishment of thought and speech. The past six decades have witnessed many commercially successful antipsychotic drugs, but no new mechanisms of action and no gains in efficacy since the early 1960s. Cognitive behavioral therapies show promise, but even when combined with current medications, individuals with schizophrenia live with profound limitations resulting from diminished control over thought, emotion, and behavior. Many pharmaceutical companies have exited psychiatry in recent years because of high failure rates in clinical trials, only rudimentary understanding of disease mechanisms, and the lack of treatment biomarkers. Under these circumstances, patients and families would have scant hope for the arrival of better drug treatments….
recent·advances·in·genomics·proteomics·and·imaging
Much about this grim scientific picture has changed in the past 5 years. New genomic technologies, combined with global collaborations to identify study participants and collect samples, have permitted the identification of a large and rapidly growing number of alleles associated with schizophrenia, bipolar disorder, and autism. Molecular pathways involved in neuronal function are emerging from the data and are beginning to suggest drug targets. Animal and in vitro models in which to investigate hundreds of gene variants of small effect remain works in progress. However, promising tools have emerged here too. For molecular and cellular analyses, stem cell technologies make possible the generation of human neurons in vitro. When combined with remarkable new genome engineering tools, these approaches permit the study of individual risk alleles, multiple alleles in molecular pathways, and the correction of risk alleles in neurons derived from patient samples. Studies at neural circuit levels are yet more challenging, but one can even envision transgenic nonhuman primate disease models with the genome engineering tools at hand. Proposals to the AMP have focused on advancing the genetic analysis of schizophrenia; improving in vitro human neuronal models to study disease-associated alleles; and a project to identify biomarkers, modeled on the early stages of the successful Alzheimer’s Disease Neuroimaging Initiative.
And now we can add in our very·important·new·phrase:
recent·exits·by·companies·from·psychiatry
Perhaps recent exits by companies from psychiatry made schizophrenia too great a reach for the AMP, despite continued strong support from NIH leadership. It is precisely when new knowledge opens challenging but real possibilities to make major advances in health that partnerships such as the AMP seem most warranted. The scientific community, including industry, academia, patient groups, and government, must find ways of sharing financial risk while developing effective and well-governed partnerships. Otherwise, important basic science investments will go untranslated while patients and society continue to bear painful and costly burdens.
Steven Hyman has been an influential figure in the development of American biomedical psychiatry. From 1996 through 2001 he was Director of the NIMH where he initiated the large drug trials [CATIE, STAR*D, etc], genetic and neuroscience research, and helped fund the early DSM-5 revision effort. In 2002 he became Provost of Harvard University, but continued his involvement with the DSM-5 and the ICD focusing on the inclusion of neuroscience and genetics. In 2011, he became the Director of the heavily endowed Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard that focuses on genetics and neuroscience research in psychiatry. He has been an active advocate of the NIMH RDoC project. The article above might best be viewed as an extension of a number of articles he’s written since moving to the Stanley Center:
by Hyman SE.
Science Translational Medicine. 2012 4[155]:155.
Drug discovery is at a near standstill for treating psychiatric disorders such as schizophrenia, bipolar disorder, depression, and common forms of autism. Despite high prevalence and unmet medical need, major pharmaceutical companies are de-emphasizing or exiting psychiatry, thus removing significant capacity from efforts to discover new medicines. In this Commentary, I develop a view of what has gone wrong scientifically and ask what can be done to address this parlous situation…
The Dana Foundation: Cerebrum
By Steven E. Hyman
April 02, 2013
PsychiatricNews
From the President
by Steven Hyman, M.D. and Jeffrey Lieberman, M.D.
October 17, 2013
by Steven E. Hyman
Neuropsychopharmacology Reviews. 2014 39:220–229.
First, my apologies for the length of this post, but I wanted to gather all of this in one place so we could think about the whole picture. So back to the phrases…
the·revolution: A lot of meanings here. One is the burst of discovery in the 1950s of medications that were effective in psychiatric conditions [lithium, antipsychotics, antidepressants, anxiolytics]. Another revolution was the coming of the neoKraepelinians and Robert Spitzer’s DSM-III in 1980. I think by the time Hyman arrived at the NINH, he might think of his tenure there as a revolution of sorts. By then, multiple classes of psychoactive drugs were flowing from the pharmaceutical pipeline at a steady rate. It was the decade of the brain, and the NIMH was front and center. Acadedemia and industry were collaborating [above and below the table]. And Hyman’s NIMH set out on a bold path to test the emerging drugs in mega clinical trials. During those years, the human genome project was completed and psychiatric genomics was becoming quite the rage. There was a feeling that the biological basis of psychiatry was just around the corner, and Hyman’s NIMH joined with the American Psychiatric Association to fund a series of symposia to plan for the next revolution, a biomedical DSM-V/5.
the·crisis: Another phrase with multiple meanings. After Hyman left the NIMH, his replacement, Tom Insel, came from a large academic/industry translational program and carried the banner of biomedical psychiatry, adding his psychiatry·as·clinical·neuroscience phrase to the base well laid by Hyman. But then came the crises. First there were a series of disillusionments as the misadventures of prominent academic psychiatrists became increasingly apparent, along with the exposure of widespread research misdemeanors in industry funded clinical trials. That culminated in Senator Grassley’s investugation of a number of high ranking psychiatrists for financial hanky-panky. Then came the legal suits exposing unmentioned adverse effects, accompanied by the release of documents that showed epidemic ghost-writing, the antics of the KOLs, the jury-rigged analyses of drug trials, and the deceitful marketing practices of PHARMA. And then there was another big crisis, the collapse of the grandiose wishes for the DSM-5 and its other foibles. But none of these are the crisis Hyman is talking about.
He’s referring to the recent·exits·by·companies·from·psychiatry that came in the summer of 2011, around the time Hyman left the Provost job for the Stanley Center – returning to the game, so to speak. The phrase I picked is Hyman’s, and it’s telling. Not recent·exits·by·companies·from·CNS·drug·development. He says recent·exits·by·companies·from·psychiatry. And I think that’s how it felt to the NIMH, to the APA, and to Steven Hyman – like they’d been abandoned by an essential ally.
So what Dr. Hyman, and Dr. Insel, and Dr. Lieberman and others at the APA say is that because of the unmet·clinical·need, the global·burden·of·disease, and the recent·advances·in·genomics·proteomics·and·imaging, this is the perfect moment to sustain the·revolution and develop new biomedical psychiatric treatments that are just·around·the·corner. But there’s the·crisis of the recent·exits·by·companies·from·psychiatry that has to be dealt with. Solutions include the NIMH et al taking on the task of drug development and compensating for the failed DSM-5 outing by creating a new diagnostic system [the RDoC] more amenable to biomedical techniques and research. Another big part of the solution is luring PHARMA back into the quest by collaboration that shares their risks in the enterprise:
"The scientific community, including industry, academia, patient groups, and government, must find ways of sharing financial risk while developing effective and well-governed partnerships."
So it’s little wonder that Dr. Hyman is disappointed to be left out of the Accelerating Medicines Partnership. It’s the kind of thing he hopes will solve the·crisis.
There’s certainly another way to look at these phrases. It’s in all the papers. In the last thirty years, psychiatry has largely equated itself with drug treatment and colluded with the PHARMA advertisement that radically inflates efficacy and downplays risk. Psychiatry has largely taken the position that all mental illness is brain disease. Over the last thirty years of this monocular biomedical psychiatry, there has developed of a huge academic·pharmaceutical complex that has functioned like a symbiosis – operating on the the capital provided by PHARMA in return for lots of things. So the recent·exits·by·companies·from·psychiatry threatens this complex with financial collapse. This second view takes into account the scientific and financial misbehavior of PHARMA and the KOLs in psychiatry. It offers a more accurate view of the medications available. And it knows that PHARMA sees the recent·advances·in·genomics·proteomics·and·imaging as offering little that’s to their advantage – likewise seeing unmet·clinical·need as well as the global·burden·of·disease for what they really are, rhetorical gimmicks. PHARMA [and the rest of the world] also knows that just·around·the·corner is a fantasy that has run out of legs.
We all know that there are many people on this planet who genuinely see the recent·exits·by·companies·from·psychiatry as the beginnings of a solution rather than the·crisis…


 and from my perspective, most of the new drugs and the activities of the KOLs were taking us nowhere. But I think Psycritic had something more profound in mind – a true designation [NIMH envisions a world in which mental illnesses are prevented and cured], a true path [driven by an ideology: the tenets of biopsychiatry], and a banner of truth. I must already agree with the banner of truth part, because some time back, I went out of my way to fabricate this graphic from a heroic poster from the days of the Russian revolution. The whole point is that quests for utiopia never get there.
and from my perspective, most of the new drugs and the activities of the KOLs were taking us nowhere. But I think Psycritic had something more profound in mind – a true designation [NIMH envisions a world in which mental illnesses are prevented and cured], a true path [driven by an ideology: the tenets of biopsychiatry], and a banner of truth. I must already agree with the banner of truth part, because some time back, I went out of my way to fabricate this graphic from a heroic poster from the days of the Russian revolution. The whole point is that quests for utiopia never get there.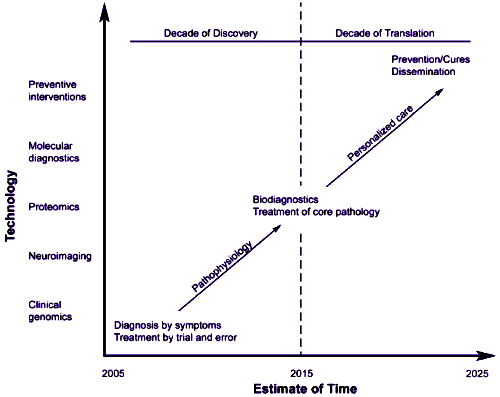
 Two years ago [on this date oddly enough], I wrote a post about tools [
Two years ago [on this date oddly enough], I wrote a post about tools [ But back then I was thinking, as I am now, about the ordinate labels on the figure above from Dr. Insel’s 2005 paper inaugurating his idea that psychiatry is, or should become, Clinical Neuroscience – genomics, neuroimaging, proteonomics, molecular diagnosis, the tools of a modern neuroscientist. I am personally a total sucker for tools. Of course I like them for what the can be used for, but it goes beyond that. I like them just for what they are. They distinguish us from other species. We don’t wait around for millennia to evolve structures to help us do new things. We make the new structures ourselves, and I’m still awed by that.
But back then I was thinking, as I am now, about the ordinate labels on the figure above from Dr. Insel’s 2005 paper inaugurating his idea that psychiatry is, or should become, Clinical Neuroscience – genomics, neuroimaging, proteonomics, molecular diagnosis, the tools of a modern neuroscientist. I am personally a total sucker for tools. Of course I like them for what the can be used for, but it goes beyond that. I like them just for what they are. They distinguish us from other species. We don’t wait around for millennia to evolve structures to help us do new things. We make the new structures ourselves, and I’m still awed by that. I first heard about those tools on the y-axis up there talked about in this way Insel is talking back in the early 1980s with the coming of a new chairman to our department. Back then, we said genetics instead of genomics, and the neuroimagers were more primitive, but the sentiment was the same. These tools were going to lead us to a new world in psychiatry. Back then I thought, as I do now, that there are some mental illnesses that were likely have a biological substrate, but certainly not all, and certainly not the ones I had come to study and learn to treat – so I exited.
I first heard about those tools on the y-axis up there talked about in this way Insel is talking back in the early 1980s with the coming of a new chairman to our department. Back then, we said genetics instead of genomics, and the neuroimagers were more primitive, but the sentiment was the same. These tools were going to lead us to a new world in psychiatry. Back then I thought, as I do now, that there are some mental illnesses that were likely have a biological substrate, but certainly not all, and certainly not the ones I had come to study and learn to treat – so I exited.  I still love tools. I spend a lot of time playing with one of the biggest ones of all – the Internet. I carry a modern tool with me whenever I venture forth from our cabin. Like everyone else, when I want to contact anyone I know in the world or find out about any topic, I expect to be able to do those things one way or another quickly – something I would never have imagined even as a younger adult. But tools are things we make to use, to explore, to communicate. They aren’t things that go on an axis of a graph. Findings go on graphs, things that tools help us discover or uncover. If you right-click on the images in this blog [leftovers from the
I still love tools. I spend a lot of time playing with one of the biggest ones of all – the Internet. I carry a modern tool with me whenever I venture forth from our cabin. Like everyone else, when I want to contact anyone I know in the world or find out about any topic, I expect to be able to do those things one way or another quickly – something I would never have imagined even as a younger adult. But tools are things we make to use, to explore, to communicate. They aren’t things that go on an axis of a graph. Findings go on graphs, things that tools help us discover or uncover. If you right-click on the images in this blog [leftovers from the