Posted on Thursday 13 March 2014
 You can’t really erase history, even though we all try. For one thing, it doesn’t go away. It just sits there in the background having an effect even if it has been selectively removed from consciousness. Freud made the analogy of The Mystic Writing Pad [when I was a kid, it was called the Magic Slate]. You wrote on cellophane with a stylus, When you lifted the cellophane, the writing disappeared, but it left traces in the wax below. Freud was using the metaphor to describe memory traces in the Unconscious.
You can’t really erase history, even though we all try. For one thing, it doesn’t go away. It just sits there in the background having an effect even if it has been selectively removed from consciousness. Freud made the analogy of The Mystic Writing Pad [when I was a kid, it was called the Magic Slate]. You wrote on cellophane with a stylus, When you lifted the cellophane, the writing disappeared, but it left traces in the wax below. Freud was using the metaphor to describe memory traces in the Unconscious.  It was a good analogy. As a psychotherapist, one learns that it’s not like in the movies – some cave full of repressed memories. But rather, the mind just skips over or goes around unpleasant or traumatic previous experience, almost without noticing. While the gain is comfort, the loss is in not learning the important lessons that experience has to teach – so history repeats. And in eliminating chapters from the story, the book-of-you makes much less sense, because we are our stories, our narrative. What else could we be?
It was a good analogy. As a psychotherapist, one learns that it’s not like in the movies – some cave full of repressed memories. But rather, the mind just skips over or goes around unpleasant or traumatic previous experience, almost without noticing. While the gain is comfort, the loss is in not learning the important lessons that experience has to teach – so history repeats. And in eliminating chapters from the story, the book-of-you makes much less sense, because we are our stories, our narrative. What else could we be?
Right now, psychiatry seems to be attempting to erase a piece of its own history – a recent piece at that. We’ve had a couple of decades where many academic psychiatrists have been in an unholy alliance with the pharmaceutical industry, one that allowed industry to control our scientific literature, our continuing medical education, and, indeed, the whole direction of our specialty.  The profits from that alliance have become the stuff of Wall Street legend – blockbusters! The ramifications of those years are everywhere around us – in our diagnostic manual, our relationships with patients and other mental health specialties, our place in the third party payment hierarchy, in the eyes of the public. As those years are finally drawing to a close, they seem to be becoming the elephant in the room that nobody’s talking about.
The profits from that alliance have become the stuff of Wall Street legend – blockbusters! The ramifications of those years are everywhere around us – in our diagnostic manual, our relationships with patients and other mental health specialties, our place in the third party payment hierarchy, in the eyes of the public. As those years are finally drawing to a close, they seem to be becoming the elephant in the room that nobody’s talking about.
-
Example: Right now, the AllTrials campaign is going great guns. Boehringer Ingelheim, GlaxoSmithKline, Roche, Sanofi, ViiV Healthcare, Pfizer, and now Johnson & Johnson are putting systems in place to allow access to their Clinical Trial data. I’ve been involved with some of the early results of that, and though it’s not a completely easy process, it’s definitely moving in the right direction towards "good enough." But nobody’s saying why they’re doing it. They’re giving us access because they’re good guys. Nobody talks about the stream of ghost written jury-rigged decepticons that flooded our literature for a decade or more, barely disguised drug industry commercials. Nobody talks about the legal settlements that are rapidly escalating to the point where they are going to really start hurting, or the growing clamor for criminal prosecutions. They’re just being generous.
-
Example: Right now, Tom Insel is renovating the NIMH. The DSM-anything is out. The RDoC is in, when it gets around to existing. NIMH Clinical Trials have been changed. We’re on a new tack to find new drug targets. The reason the DSM-5 is out? It’s because medications and neuroscience findings don’t map onto the clinical categories. Little is mentioned about the lackey-ing around with drug trials and neuroimaging/genetics/etc. work the NIMH has funded to study and cavort with the industry’s drug output. Nothing is said about the APA/NIMH series of symposia in the lead up to the DSM-5. Not much mentioned that the Research Agenda for the DSM-V essentially laid out the RDoC agenda which came into being as it became apparent that the grand plans for the DSM-5 were going up in smoke. And there’s absolutely no comment about the fact that nothing [not even patients] map well to the DSM because it has been so distorted by outside forces.
-
Example: Dr. Lieberman [APA President] and now Dr. Summergrad [APA President Elect] can’t talk enough about something called Collaborative Psychiatry – meaning psychiatrists should work in practices with general physicians. But they don’t mention that psychiatry so bought into the psychiatrists-as-medication-prescribers model and now there are no more new meds to prescribe that they’re trying to find some kind of new identity for psychiatrists to fit into.
We did this already in 1980 – abandoned our history, whether by intent or not. One would’ve thought that the only historical figure that ever mattered was Emil Kraepelin. The psychoanalysts, Adolf Meyers, Harry Stack Sullivan, Karl Jaspers, social psychiatrists, family theorists, psychotherapists [other that CBTers] – the pantheon of psychiatrists who had contributed to our understanding of mental illness were largely forgotten and rarely mentioned in any positive way. And of interest, since the 1980s we haven’t produced any "greats" – only KOLs with a limited shelf-life.


 An ancient reaction to fear, distress, and calamity has been to rely on religion. “When cause and cure are unknown, magic and religion supply welcome hope”. In biblical times, mental illness was seen as the opposite of what was “good.” During the Middle Ages, most progress in medical science was severely squelched. The Christian church, consumed with superstition and demonic possession, rode herd on the diagnosis and treatment of mental illness. During the Renaissance, an obsession with evil in the form of witches became prominent. The official practice guidelines on detecting witches, the Malleus Maleficarum [1486], assisted inquisitors in finding evil lurking amid women, the socially disenfranchised, and the mentally ill.
An ancient reaction to fear, distress, and calamity has been to rely on religion. “When cause and cure are unknown, magic and religion supply welcome hope”. In biblical times, mental illness was seen as the opposite of what was “good.” During the Middle Ages, most progress in medical science was severely squelched. The Christian church, consumed with superstition and demonic possession, rode herd on the diagnosis and treatment of mental illness. During the Renaissance, an obsession with evil in the form of witches became prominent. The official practice guidelines on detecting witches, the Malleus Maleficarum [1486], assisted inquisitors in finding evil lurking amid women, the socially disenfranchised, and the mentally ill.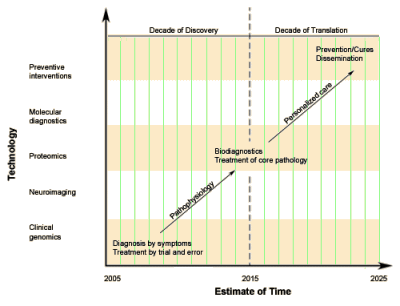
 Tom Insel stepped from his training into the intramural program at the NIMH where he spent a decade and a half doing research on SSRIs and the effect of hormones on behavior. In the early 90s, he moved to direct the Yerkes Primate lab and later a Translational Research program, both in the Department chaired by Dr. Charlie Nemeroff. It was the decade of the brain, and Emory/Atlanta was an epicenter for matters brain and drug. That orientation followed him to the NIMH where his directive style and bent towards Clinical Neuroscience has created a cohort of NIMH Grantees that have dutifully followed his direction.
Tom Insel stepped from his training into the intramural program at the NIMH where he spent a decade and a half doing research on SSRIs and the effect of hormones on behavior. In the early 90s, he moved to direct the Yerkes Primate lab and later a Translational Research program, both in the Department chaired by Dr. Charlie Nemeroff. It was the decade of the brain, and Emory/Atlanta was an epicenter for matters brain and drug. That orientation followed him to the NIMH where his directive style and bent towards Clinical Neuroscience has created a cohort of NIMH Grantees that have dutifully followed his direction. We often use cracks as a metaphor: unwanted things [like weeds] shooting up through the cracks or things disappearing by falling through the cracks. The last post [
We often use cracks as a metaphor: unwanted things [like weeds] shooting up through the cracks or things disappearing by falling through the cracks. The last post [ But cracks go both ways, letting things in and out. And one thing about things falling through the cracks, you don’t necessarily see it happen. You don’t know that something was there and then disappeared, it’s an empty space that doesn’t show. With snowfall on a deck, you can see the absences – the cracks. With pharmaceutical clinical trials, unless you know how to look, unpublished trials aren’t apparent.
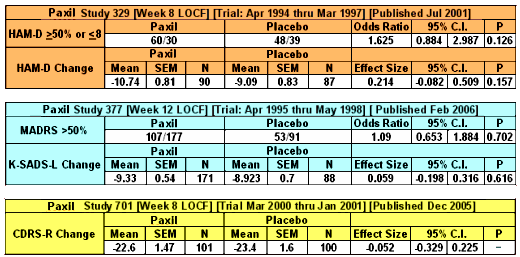
But cracks go both ways, letting things in and out. And one thing about things falling through the cracks, you don’t necessarily see it happen. You don’t know that something was there and then disappeared, it’s an empty space that doesn’t show. With snowfall on a deck, you can see the absences – the cracks. With pharmaceutical clinical trials, unless you know how to look, unpublished trials aren’t apparent.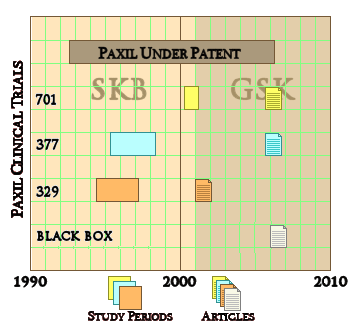 SmithKlineBeecham/GlaxoSmithKline gave us a classical example with the infamous Paxil® Study 329. They actually did three Clinical Trials [701, 377, 329] on Paxil® in adolesence, all completed before study 329 was published in 2001. That was the reprint handed out by the reps, promoting "off-label" use. The other two studies [701 & 377] were published after Paxil went off-patent 5 years later [as a CYA move]. Those studies were so negative that no amount of jury-rigging could save them. So, through the cracks they tumbled.
SmithKlineBeecham/GlaxoSmithKline gave us a classical example with the infamous Paxil® Study 329. They actually did three Clinical Trials [701, 377, 329] on Paxil® in adolesence, all completed before study 329 was published in 2001. That was the reprint handed out by the reps, promoting "off-label" use. The other two studies [701 & 377] were published after Paxil went off-patent 5 years later [as a CYA move]. Those studies were so negative that no amount of jury-rigging could save them. So, through the cracks they tumbled. To my way of thinking, cracks in regulations have been of major import to the whole history of deceptive marketing practices of the pharmaceutical industry. David Healy’s under-read
To my way of thinking, cracks in regulations have been of major import to the whole history of deceptive marketing practices of the pharmaceutical industry. David Healy’s under-read