Probably the reason that you don’t know about TORDIA [Treatment Of Resistant Depression In Adolescents] is that it was a doomed ship – cursed by fatal design and haunted by a storm of fate. By now you know that the new century of psychopharmacology opened with an all out assault on a new mental illness called treatment resistant depression, spearheaded by a gaggle of initialed studies by Steve Hyman’s NIMH. The afflicted were people diagnosed with Major Depressive Disorder whose symptoms stubbornly resisted the should-cure-everyone SSRIs. There was an almost universal conviction that there was some clever manipulation of the various drugs, some algorithm, that would extend their [then inflated] efficacy. It’s the sentiment that produced TMAP, STAR*D, IMPACT, COMED, and lives on in EMBARC – and in the child and adolescent realm, it produced TADS and TORDIA.
TORDIA was supported with NIMH grants MH61835, MH61856, MH61864, MH61869, MH61958, and MH62014, and MH66371 – one for each site. The study itself ran from 2001 through 2006. The reason I said it was cursed by poor design is that there was no control group and there were a plethora of heterogeneous experimental groups. The reason I said it was haunted is that Paxil was taken off the market in the UK and the black box warning was issued in the US during the study, so they had to reshuffle the protocols to adapt to new guidelines. Like STAR*D, TORDIA produced a lot of publications with an army of authors. The two main efficacy reports are here:
by David Brent, MD; Graham Emslie, MD; Greg Clarke, PhD; Karen Dineen Wagner, MD, PhD; Joan Rosenbaum Asarnow, PhD; Marty Keller, MD; Benedetto Vitiello, MD; Louise Ritz, MBA; Satish Iyengar, PhD; Kaleab Abebe, MA; Boris Birmaher, MD; Neal Ryan, MD; Betsy Kennard, PsyD; Carroll Hughes, PhD; Lynn DeBar, PhD; James McCracken, MD; Michael Strober, PhD; Robert Suddath, MD; Anthony Spirito, PhD; Henrietta Leonard, MD; Nadine Melhem, PhD; Giovanna Porta, MS; Matthew Onorato, LCSW; and Jamie Zelazny, MPH, RN
JAMA. 2008 299[8]:901-913.
Context: Only about 60% of adolescents with depression will show an adequate clinical response to an initial treatment trial with a selective serotonin reuptake inhibitor [SSRI]. There are no data to guide clinicians on subsequent treatment strategy.
Objective: To evaluate the relative efficacy of 4 treatment strategies in adolescents who continued to have depression despite adequate initial treatment with an SSRI.
Design, Setting, and Participants: Randomized controlled trial of a clinical sample of 334 patients aged 12 to 18 years with a primary diagnosis of major depressive disorder that had not responded to a 2-month initial treatment with an SSRI, conducted at 6 US academic and community clinics from 2000-2006.
Interventions: Twelve weeks of: [1] switch to a second, different SSRI [paroxetine, citalopram, or fluoxetine, 20-40 mg]; [2] switch to a different SSRI plus cognitive behavioral therapy; [3] switch to venlafaxine [150-225 mg]; or [4] switch to venlafaxine plus cognitive behavioral therapy.
Main Outcome Measures: Clinical Global Impressions-Improvement score of 2 or less [much or very much improved] and a decrease of at least 50% in the Children’s Depression Rating Scale-Revised [CDRS-R]; and change in CDRS-R over time.
Results: Cognitive behavioral therapy plus a switch to either medication regimen showed a higher response rate [54.8%; 95% confidence interval [CI], 47%-62%] than a medication switch alone [40.5%; 95% CI, 33%-48%; P = .009], but there was no difference in response rate between venlafaxine and a second SSRI [48.2%; 95% CI, 41%-56% vs 47.0%; 95% CI, 40%-55%; P = .83]. There were no differential treatment effects on change in the CDRS-R, self-rated depressive symptoms, suicidal ideation, or on the rate of harm-related or any other adverse events. There was a greater increase in diastolic blood pressure and pulse and more frequent occurrence of skin problems during venlafaxine than SSRI treatment.
Conclusions: For adolescents with depression not responding to an adequate initial treatment with an SSRI, the combination of cognitive behavioral therapy and a switch to another antidepressant resulted in a higher rate of clinical response than did a medication switch alone. However, a switch to another SSRI was just as efficacious as a switch to venlafaxine and resulted in fewer adverse effects.
Note: I said in the last post [off the science grid…] that I didn’t know where Dr. Wagner got "…60% of youngsters will respond favorably to their first antidepressant medication – generally a selective serotonin reuptake inhibitor (SSRI)." Well there it is under Context. This article is available full text online and that line is marked with references 12 through 16 which are linked. Take a look and see if you can get 60% out of them. I can’t.
What they did was gather a cohort of 334 subjects that had been SSRI non-responders and switched their medications to another drug. This was a time when the differences among the SSRIs and the difference between SSRIs and SNRIs was thought to matter, at least more than now. So they switched half to a different SSRI and half to an SNRI. Likewise, this was a time when CBT [Cognitive Behavior Therapy] was treated as something like a drug, so they further partitioned the two switched groups, half getting CBT and the other not. At 12 weeks, they had a 47/48% response to changing drugs, and it didn’t matter to which drug. And adding CBT pushed it up to 55%. What we don’t know is what would’ve happened had they been continued on their original drug; or given a placebo; or treated with CBT alone. So there’s no denominator in any equation. Like in STAR*D, the implication was that subsequent treatments were value added at a lower efficacy rate – but without controls, those conclusions remain, at best, speculations rather than true outcomes.
by Graham J. Emslie, M.D.; Taryn Mayes, M.S.; Giovanna Porta, M.S.; Benedetto Vitiello, M.D.; Greg Clarke, Ph.D.; Karen Dineen Wagner, M.D., Ph.D.; Joan Rosenbaum Asarnow, Ph.D.; Anthony Spirito, Ph.D.; Boris Birmaher, M.D.; Neal Ryan, M.D.; Betsy Kennard, Psy.D.; Lynn DeBar, Ph.D.; James McCracken, M.D.; Michael Strober, Ph.D.; Matthew Onorato, L.C.S.W.; Jamie Zelazny, M.P.H., R.N.; Marty Keller, M.D.; Satish Iyengar, Ph.D.; David Brent, M.D.
American Journal of Psychiatry 2010 167:782-791.
Objective: The purpose of this study was to report on the outcome of participants in the Treatment of Resistant Depression in Adolescents [TORDIA] trial after 24 weeks of treatment, including remission and relapse rates and predictors of treatment outcome.
Method: Adolescents [ages 12—18 years] with selective serotonin reuptake inhibitor [SSRI]-resistant depression were randomly assigned to either a medication switch alone [alternate SSRI or venlafaxine] or a medication switch plus cognitive-behavioral therapy [CBT]. At week 12, responders could continue in their assigned treatment arm and nonresponders received open treatment [medication and/or CBT] for 12 more weeks [24 weeks total]. The primary outcomes were remission and relapse, defined by the Adolescent Longitudinal Interval Follow-Up Evaluation as rated by an independent evaluator.
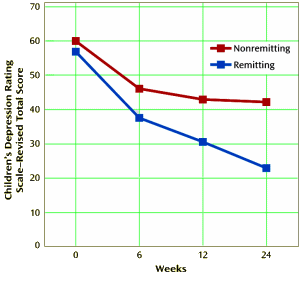
Results: Of 334 adolescents enrolled in the study, 38.9% achieved remission by 24 weeks, and initial treatment assignment did not affect rates of remission. Likelihood of remission was much higher [61.6% versus 18.3%] and time to remission was much faster among those who had already demonstrated clinical response by week 12. Remission was also higher among those with lower baseline depression, hopelessness, and self-reported anxiety. At week 12, lower depression, hopelessness, anxiety, suicidal ideation, family conflict, and absence of comorbid dysthymia, anxiety, and drug/alcohol use and impairment also predicted remission. Of those who responded by week 12, 19.6% had a relapse of depression by week 24.
Conclusions: Continued treatment for depression among treatment-resistant adolescents results in remission in approximately one-third of patients, similar to adults. Eventual remission is evident within the first 6 weeks in many, suggesting that earlier intervention among nonresponders could be important.
After 12 weeks, there was yet another division among the subjects. Responders could continue their current treatment. Non-responders could receive "open treatment" – something like dealers choice by unrestrained clinicians. 20% of the 12 week responders relapsed, but as a group kept improving. And "open treatment" didn’t do anything for the 12 week non-responders. In kids, the third tier didn’t add anything.
Along the way, Paxil was withdrawn, Effexor was cautioned, there was a black box warning added to all of the drugs in play, and around publication time, Senator Grassley lowered the boom on a couple of the authors [including Wagner]. TORDIA slipped under the waves in spite of a
publishing flurry [listed on clinicaltrials.gov at the end]. The TORDIA revival mentioned in the last post [
off the science grid…] actually started two year’s ago with a paper lead by Dr. Wagner [
timeline of woes included]:
by Wagner KD, Asarnow JR, Vitiello B, Clarke G, Keller M, Emslie GJ, Ryan N, Porta G, Iyengar S, Ritz L, Zelanzny J, Onorato M, and Brent D.
Journal of Child and Adolescent Psychopharmacology. 2012 22[1]:5-10.
OBJECTIVE: The purpose of this article is to describe the effects of the pediatric antidepressant controversy on the Treatment of Serotonin-Selective Reuptake Inhibitor [SSRI] Resistant Depression in Adolescents [TORDIA] trial.
METHOD: Adolescents, ages 12-18 years, with SSRI resistant depression were randomized to one of four treatments for a 12 week trial: Switch to different SSRI, switch to an alternate antidepressant [venlafaxine], switch to an alternate SSRI plus cognitive behavior therapy [CBT], or switch to venlafaxine plus CBT.
RESULTS: The health advisories and "black box" warnings regarding suicidality and antidepressants in adolescents occurred during the course of the TORDIA trial. Revisions to the protocol, multiple-consent form changes, and re-consenting of patients were necessary. Recruitment of participants was adversely affected.
CONCLUSION: Despite a cascade of unforeseen events that delayed the completion of the study, the TORDIA trial resulted in clinically important information about treatment-resistant depression in adolescents.
Rather than be direct, I’ll make my editorial comments about this study with an analogy that spontaneously occurred to me while writing my last post:
 Half a life ago, we were on a long drive-about in Scandanavia loaded with the guidebooks Americans carry everywhere, and came across a reference to a site we’d never heard of – the VASA, a wooden tall ship built in the 17th century that had been salvaged from the briny deep. She was indeed a site to remember. At the time we saw her, she was in a temporary building constantly, bathed with a saline spray to preserve the wood. She was largely intact – a trip into that part of history like no other. Being a guidebook scanner rather than reader, I was well into our visit to the ship before I heard the whole story. Minutes after being launched on her maiden voyage in 1628, the first gust of wind of note capsized the boat and she sank in the briny river harbor [thus the preservation]. The Vasa was reclaimed in 1961 and now has a very popular museum of her own in Stockholm.
Half a life ago, we were on a long drive-about in Scandanavia loaded with the guidebooks Americans carry everywhere, and came across a reference to a site we’d never heard of – the VASA, a wooden tall ship built in the 17th century that had been salvaged from the briny deep. She was indeed a site to remember. At the time we saw her, she was in a temporary building constantly, bathed with a saline spray to preserve the wood. She was largely intact – a trip into that part of history like no other. Being a guidebook scanner rather than reader, I was well into our visit to the ship before I heard the whole story. Minutes after being launched on her maiden voyage in 1628, the first gust of wind of note capsized the boat and she sank in the briny river harbor [thus the preservation]. The Vasa was reclaimed in 1961 and now has a very popular museum of her own in Stockholm.
It remains a wonderful irony that by far the best relic we have of the age of tall ships is the Vasa, a ship whose design was so flawed that she sailed less than a mile before sinking in a light wind. She was revived after centuries and stands as a peculiar testimony to bygone days…



 I couldn’t have hoped for a more direct answer than that. The data itself is to be in the hands of YODA, and they would see replicating results as an acceptable research question. That is Data Transparency. They reserve the right to insist on a qualified team that agrees to stick to the submitted question. Those are expectable hoops and within their right to request.
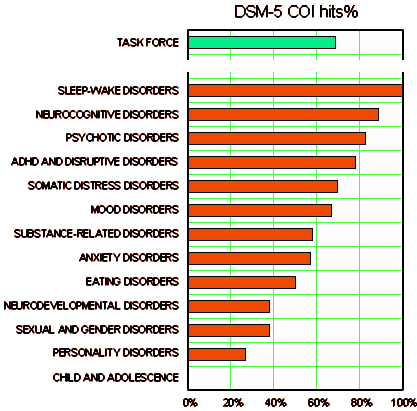
I couldn’t have hoped for a more direct answer than that. The data itself is to be in the hands of YODA, and they would see replicating results as an acceptable research question. That is Data Transparency. They reserve the right to insist on a qualified team that agrees to stick to the submitted question. Those are expectable hoops and within their right to request. It has appeared to me that the die was cast for the DSM-5 long before the revision process proper was ever underway. I’ve repeatedly mentioned the book A Research Agenda for DSM-V – the 2002 book that set the stage for two major conceptual changes: the inclusion of biological parameters and the addition of dimensional diagnostic criteria. That book was followed by a series of symposiums. While dimensional diagnosis was discussed in many of those conferences, there was one devoted to dimensional diagnosis specifically, and it was published as a separate book in 2007,
It has appeared to me that the die was cast for the DSM-5 long before the revision process proper was ever underway. I’ve repeatedly mentioned the book A Research Agenda for DSM-V – the 2002 book that set the stage for two major conceptual changes: the inclusion of biological parameters and the addition of dimensional diagnostic criteria. That book was followed by a series of symposiums. While dimensional diagnosis was discussed in many of those conferences, there was one devoted to dimensional diagnosis specifically, and it was published as a separate book in 2007, 


 What a thoughtful piece about the illusion of scientific detachment. The ancient Greeks thought if they could only master the rules of logic, enumerate all the logical fallacies, they would be able to reach absolute truths. They were the Dogmatists [back in a time when it meant the search for truth rather than what dogmatic means now]. With logic – every single day lawyers joust in courtrooms with carefully crafted legal arguments whose logic impeccably proves opposite points in the same case [and leaves the choice up to the everymen in the jury]. In science, we have our own versions a bit beyond simple logic with our data and its surrogates neatly arrayed in tables and graphs leading to conclusions wrapped in a cloak of statistical and mathematical proof.
What a thoughtful piece about the illusion of scientific detachment. The ancient Greeks thought if they could only master the rules of logic, enumerate all the logical fallacies, they would be able to reach absolute truths. They were the Dogmatists [back in a time when it meant the search for truth rather than what dogmatic means now]. With logic – every single day lawyers joust in courtrooms with carefully crafted legal arguments whose logic impeccably proves opposite points in the same case [and leaves the choice up to the everymen in the jury]. In science, we have our own versions a bit beyond simple logic with our data and its surrogates neatly arrayed in tables and graphs leading to conclusions wrapped in a cloak of statistical and mathematical proof.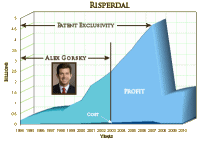 It feels like we’re only going to have one shot at Data Transparency, and we need to get it right. And at least in the realm of psychoactive medications, the level of misbehavior by the pharmaceutical industry is the stuff of legend. When and if the history is ever written, Johnson & Johnson will probably have a whole chapter all to themselves. The TMAP Program in Texas alone would qualify them, but there were other things including the nearby Excerpta Medica that ghost wrote Risperdal
It feels like we’re only going to have one shot at Data Transparency, and we need to get it right. And at least in the realm of psychoactive medications, the level of misbehavior by the pharmaceutical industry is the stuff of legend. When and if the history is ever written, Johnson & Johnson will probably have a whole chapter all to themselves. The TMAP Program in Texas alone would qualify them, but there were other things including the nearby Excerpta Medica that ghost wrote Risperdal

 Half a life ago, we were on a long drive-about in Scandanavia loaded with the guidebooks Americans carry everywhere, and came across a reference to a site we’d never heard of – the VASA, a wooden tall ship built in the 17th century that had been salvaged from the briny deep. She was indeed a site to remember. At the time we saw her, she was in a temporary building constantly, bathed with a saline spray to preserve the wood. She was largely intact – a trip into that part of history like no other. Being a guidebook scanner rather than reader, I was well into our visit to the ship before I heard the whole
Half a life ago, we were on a long drive-about in Scandanavia loaded with the guidebooks Americans carry everywhere, and came across a reference to a site we’d never heard of – the VASA, a wooden tall ship built in the 17th century that had been salvaged from the briny deep. She was indeed a site to remember. At the time we saw her, she was in a temporary building constantly, bathed with a saline spray to preserve the wood. She was largely intact – a trip into that part of history like no other. Being a guidebook scanner rather than reader, I was well into our visit to the ship before I heard the whole