Posted on Monday 6 January 2014
Committee of Public Accounts,
June 2013
Evidence Based Medicine [often pronounced evidencebasedmedicine] has evolved into one of my least favorite terms, even beating out chemicalimbalance. The reason it tops the list is that it’s even used by people who are not tricksters like the chemicalimbalance set. They often mean well. They’re saying that you shouldn’t confuse opinion or preference with fact, that too many people in this world render expert medical opinions or advice that aren’t backed up by anything factual – and that’s an excellent point. There’s a background implication that people in medicine who do this shoot-from-the-hip thing are charlatans with ulterior motives. So if I’m extolling the virtues of evidencebasedmedicine, why is it my least favorite term? Well, part of the answer is PTSD. If you were in psychoanalytic training in the late 1970s or early 1980s, you would’ve been bludgeoned with "what is the evidence for…?" questions or been seen as a necromancer or worse so many times that you wince at the mention. Being a psychiatrist wasn’t much better with the Szaszian critiques or the reductionistic-medical-model-thinking criticisms that seemed to come out between the bricks and through the cracks in the windows. These days, it’s being put in the position of defending the belief that all mental illness is brain disease treatable with toxic chemicals. I’m obviously really not very good at that one. So, to summarize, many people use the term evidencebasedmedicine to say, "You’re a deluded lunatic."
But that’s not why it’s my least favorite term. It’s because it has been turned into a trick, a surrogate for the scientific method. And it has become an agent of harm. Three years ago, I drew the picture on the left to diagram EMB as it was being presented in a particular article: Structured Interview, Diagnostic Manual, Treatment Algorithm informed by Clinical Trials, resultant Treatment recommendation based on EBM treatments. On the right, I’ve circled the elements that have been regularly misused in one or another form of corruption. Every one of them vulnerable to misuse by the prophets of evidencebasedmedicine:
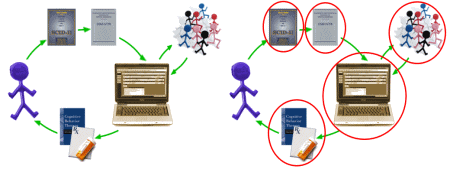
In fact, there’s really only one kind of Medicine, and it’s Evidence Based Medicine. But many now use evidencebasedmedicine as a false certification of their distortions due to conflictsofinterest. That’s my introduction to this article:
by Des SpenceBritish Medical Journal. 2014 348:doi: [Published 3 January 2014]Evidence based medicine [EBM] wrong footed the drug industry for a while in the 1990s. We could fend off the army of pharmaceutical representatives because often their promotional material was devoid of evidence. But the drug industry came to realise that EBM was an opportunity rather than a threat. Research, especially when published in a prestigious journal, was worth more than thousands of sales representatives. Today EBM is a loaded gun at clinicians’ heads. “You better do as the evidence says,” it hisses, leaving no room for discretion or judgment. EBM is now the problem, fueling overdiagnosis and overtreatment.
You see, without so called “evidence” there is no seat at the guideline table. This is the fundamental “commissioning bias,” the elephant in the room, because the drug industry controls and funds most research. So the drug industry and EBM have set about legitimising illegitimate diagnoses and then widening drug indications, and now doctors can prescribe a pill for every ill. The billion prescriptions a year in England in 2012, up 66% in one decade, do not reflect a true increased burden of illness nor an ageing population, just polypharmacy supposedly based on evidence. The drug industry’s corporate mission is to make us all sick however well we feel. As for EBM screening programmes, these are the combine harvester of wellbeing, producing bails of overdiagnosis and misery.
Corruption in clinical research is sponsored by billion dollar marketing razzmatazz and promotion passed off as postgraduate education. By contrast, the disorganised protesters have but placards and a couple of felt tip pens to promote their message, and no one wants to listen to tiresome naysayers anyway.
How many people care that the research pond is polluted, with fraud, sham diagnosis, short term data, poor regulation, surrogate ends, questionnaires that can’t be validated, and statistically significant but clinically irrelevant outcomes? Medical experts who should be providing oversight are on the take. Even the National Institute for Health and Care Excellence and the Cochrane Collaboration do not exclude authors with conflicts of interest, who therefore have predetermined agendas. The current incarnation of EBM is corrupted, let down by academics and regulators alike.
What do we do? We must first recognise that we have a problem. Research should focus on what we don’t know. We should study the natural history of disease, research non-drug based interventions, question diagnostic criteria, tighten the definition of competing interests, and research the actual long term benefits of drugs while promoting intellectual scepticism. If we don’t tackle the flaws of EBM there will be a disaster, but I fear it will take a disaster before anyone will listen.
A recent post on Behaviorism and Mental Health, Psychiatry’s Over Reliance On Pharma, took a meta-look at Dr. Lieberman’s The NIMH-CATIE Schizophrenia Study: What Did We Learn? Dr. Hickey pointed to Dr. Lieberman’s blaming pharma for their distorted messages, rather than holding psychiatrists responsible for listening to those messages, and continuing to listen even now after all the scandal. Fair point, well made. But there was a discussion in the comments about clinical trials that brought back some old memories. They have to do with calling clinical trials of drugs research. I don’t think of clinical drug trials as research. I think of them as product testing.
In my case, the DSM-III revolution arrived in the form of a person, a new chairman, and he spoke of nothing except research. At first, I thought he was interested in building the department into a more academic place, and maybe that was right. But as I listened, what he primarily meant was clinical drug research. For that and a thousand other reasons, I began to look for other work. That’s a long story that’s not pertinent here. What is pertinent is that I had left a research tract in an earlier time of life, not in psychiatry, and I had a fairly solid idea what research was. Drug trials were an aside, something reasonably important, but not research. At some point, part of this department building thing was to institute a monthly Grand Rounds with outside speakers. The first one was a presentation by someone who gave a drug talk about Mellaril, paid for by Sandoz [that speaker was later the first author on the primo Seroquel clinical trial and even later spent ten years in prison for fraud]. I was gone into private practice not too long after that Grand Rounds.
By any right-thinking that I know, a clinical drug trial is essentially composed of two parts: the design, which specifies all the details in a protocol about how it is to be conducted and analyzed; and the trial itself, which is carried out according to that protocol including the result analysis at the end [predefined]. The whole point of the pre·definition is to curb the temptation to play with the data once it is in hand. I would see that not as a research endeavor, but as administrative enterprise – something important to do well, but more in the range of robotic than creative. I hate to use the term assembly line because it has negative connotations and I don’t mean that, but I can’t think of something else – so "good assembly line" is the point here. And one needs to be paid well to do it, because it’s a pain in the ass to do. Part of the drill is to treat all subjects the same, and that’s hard to do in clinical medicine with sick people.
Everything about a clinical drug trial design is an attempt to eliminate bias, human and otherwise: randomization, blinding, standardized instruments, placebo control, active comparators, an a priori protocol, etc. Creativity, following hunches, making outside observations, anything that branches from the straight line is verboten – antithetical to the point of the trial. If something interesting happens along the way, apply for another grant because it doesn’t belong in this one. Research is something else. Research has branches. A hypothesis or a question is where it starts, but it’s refined, or redirected, or even abandoned along the way. It’s more about immersion in a question that’s unanswered and looking all around for a way out, like a spot of mold on the petri dish. In a clinical trial, that’s a contaminated sample, missing data. In research, it’s the discovery of penicillin.
At least in psychiatry, those invested in the outcome of a clinical trial have found a number of ways to lean the results. Sometimes it’s in the design e.g. an active comparator given in the wrong dose. But usually it’s by adding a third part – playing with the results to see if they lend themselves to presentation enhancement techniques; or re·framing adverse effects; or putting the whole thing in a file drawer to grow old alone. A lot of the KOLs who sign these things really don’t involve themselves in that process it turns out. They’re just tickets into an academic journal or window dressing for the author’s byline. Five or six years ago, I didn’t actually know that. They’re called researchers, but they aren’t, and many aren’t even trialists. They’re more like trophy·wives or front·men. They know a lot and present well, but they’re something other than researchers or trialists.
So back to Phil’s point. Why have psychiatrists continued to listen? Why psychiatry’s over·reliance on pharma? Part of the real answer is that they didn’t know it was just pharma. Another part is that there was no other place to listen. Another part is that their patients watch too much television. Another part is that’s what they get paid to do. Another part is that’s what they get referred patients for – "med consults." Another part is it’s easy. And the worst part is that they don’t put the time and effort into swimming upstream and figuring things out for themselves. I actually liked the CATIE study even if I’m not so taken with Dr. Lieberman. Maybe the overly-positive messages about the Atypical Antipsychotics did ultimately come from pharma, but they came out of the mouths of academic psychiatrists and flowed into algorithms and guidelines certified by same that were everywhere.
Back to the beginning and my memories. I have been reassured by many who were there to know that I trust that the pharmaceutical invasion of academic/practicing psychiatry came after the DSM-III revolution and did not have a finger in causing it. I have to take that on faith, because in my microcosm, they came at the same time. And there were a number of young psychiatrists who came to Atlanta [from Saint Louis] who opened clinical research centers in that same time frame [that are still going strong]. For that matter, John Feighner, the Saint Louis psychiatry resident whose criteria formed the nucleous of the DSM-III went on to have a successful and lucrative career running a clinical research center. So, at the least, the synergy between a segment of psychiatry and pharmaceutical industry funded clinical drug trials came early on in the days of the DSM-III, certainly in my neck of the woods.
I think Deep Throat’s line, "Follow the money," was a bit of fiction from screenwriter William Goldman rather than historical fact, but it doesn’t matter. It captured a principle that may well outlive its origins. Here, Ginny Barbour uses it with finesse:
PLoS blogsBy Virginia BarbourJanuary 3, 2014Ginny Barbour, Medicine Editorial Director at PLOS, discusses a recently-published UK Government spending report on access to clinical trial information and the stockpiling of Tamiflu.
Yesterday the UK Parliament’s Public Accounts Committee, which monitors UK Government spending – focusing on “value-for-money criteria which are based on economy, effectiveness and efficiency” – published a rather amazing report; not just because of the topic of its enquiry but in particular its conclusions, which have implications well beyond the UK. The topic was “Access to clinical trial information and the stockpiling of Tamiflu”: the conclusions were that the money spent on Tamiflu was likely misspent, and moreover the lack of access to clinical trial data more widely is unacceptable.Richard Bacon MP, member of the Committee of Public Accounts, said in quote which succinctly sums up how unacceptable the current position is :
“The full results of clinical trials are being routinely and legally withheld from doctors and researchers by the manufacturers of medicines. This has ramifications for the whole of medicine. The ability of doctors, researchers and patients to make informed decisions about treatments is being undermined. Regulators and the industry have recently made proposals to open up access, but these do not cover the issue of access to the results of trials in the past which bear on the efficacy and safety of medicines in use today.”Or, as the report’s summary says
“This longstanding regulatory and cultural failure impacts on all of medicine, and undermines the ability of clinicians, researchers and patients to make informed decisions about which treatment is best.”AllTrials, a coalition spearheaded by Ben Goldacre [doctor and author of Bad Pharma], the charity Sense About Science, the BMJ, PLOS [where I am the Medicine Editorial Director] and a group of independent academics, was born out of real anger that the many backroom discussions on trials were simply going nowhere and there was a need to bring the issue of hidden data into the public view. The campaign has been successful in mobilizing academics and patients groups, has lobbied UK and European Parliamentarians and overall has been a key reason in the movement toward more transparency of a number of pharmaceutical companies.It’s ironic in the end though that it may take a Committee whose job it is to look at spending to point out what Health Departments seems to have been willing to ignore – that hiding clinical trial data is tremendously damaging to society, at an individual, professional and yes, even a financial level.
Ben Goldacre sums it up thus:
“We cannot make informed decisions about which treatment is best, when vitally important information is routinely and legally kept secret. Future generations will look back at this absurd situation in the same way that we look back on mediaeval bloodletting.”
 It’s not really so ironic, it’s the way the world works. Allen Jones ran across a funny account working as an investigator for the Pennsylvania OIG. The trail he followed ended up busting a mega-scam called TMAP, still under-prosecuted. How could we be surprised that it was the money spent by the British government on Tamiflu that finally revealed that the efficacy of the drug rested on selected clinical trials, and there were a raft of unpublished trials sitting in Roche’s file drawers? Joel Grey and Liza Manelli told us that "Money makes the world go round" a long time ago. Thus, the Pharmaceutical industry and friends have tainted the integrity of the medical profession as a whole keeping it spinning…
It’s not really so ironic, it’s the way the world works. Allen Jones ran across a funny account working as an investigator for the Pennsylvania OIG. The trail he followed ended up busting a mega-scam called TMAP, still under-prosecuted. How could we be surprised that it was the money spent by the British government on Tamiflu that finally revealed that the efficacy of the drug rested on selected clinical trials, and there were a raft of unpublished trials sitting in Roche’s file drawers? Joel Grey and Liza Manelli told us that "Money makes the world go round" a long time ago. Thus, the Pharmaceutical industry and friends have tainted the integrity of the medical profession as a whole keeping it spinning…
| AGENCY | SITE | Wikipedia |
|
|
||
| NICE | <link> | <link> |
| MHRA |
<link> | <link> |
| EMA | <link> | <link> |
So I changed my mind. This is the clinical trials part of the report mentioned in the last post [a river…] by the House of Commons Committee of Public Accounts [Access to clinical trial information and the stockpiling of Tamiflu]. The UK system is a National Health Service so the Agency structure is different. On the right are some links for the ones mentioned. It’s a different system, but the problems are the same. NICE and MHRA are UK Agencies and EMA is a European Union Agency.
I’m not sure why, but this feels like a story that needs a history to go along with it. In August 2012, I was wandering the Internet looking for I-don’t-recall-what, and I landed on the GSK site where I found something I hadn’t seen before – all the raw data from Paxil Study 329. Like many, I had been mildly obsessed with that study ever since running across Jon Juriedini‘s 2003 letter, and I knew that data hadn’t been there before [a movement…]. I wrote David Healy thinking that if anyone might know why it was there, he would. And he did, telling me that Peter Doshi, an epidemiologist, had gotten GSK to publish it a week or so before. Apparently, that was part of the settlement that Elliot Spitzer got in 2004, but they’d only published summaries until pressed by Doshi. I didn’t know who Peter Doshi was, but quickly found that he and colleague Tom Jefferson were running down the Tamiflu story. Governments were spending billions stockpiling Tamiflu in case of a flu epidemic, but the Cochrane group found that there were many missing trials and Roche wouldn’t release them. Here are some references from their work [Drug Data Shouldn’t Be Secret, The Imperative to Share Clinical Study Reports: Recommendations from the Tamiflu Experience]. That was my introduction to the movement that ultimately became the AllTrials petition and campaign. Back then, I stuck with having a shot at the Paxil Study 329 data [starting with the lesson of Study 329: the basics…].
"Unless we can find a solution to the commercial incompetence problem, we have to recognize that the pharmaceutical industry has an irreducible conflict of interest in relation to the way it represents its drugs, in science and in marketing. And unless we can resolve this in a way that is more in the public interest and in patients’ interest, I would argue that drug companies should not be allowed to evaluate their own products."
The Carlat Psychiatry Blogby Danny CarlatJune 30, 2009
Psychiatry’s diagnostic manual is due for a revision. But what began as a group of top scientists reviewing the research literature has degenerated into a dispute that puts the Hatfield-McCoy feud to shame. The latest installment in this remarkable episode of American psychiatry involves an editorial by Dr. Allen Frances, the chairman of the committee that created the current version of the the DSM, the DSM-IV. The editorial has not even been officially published [it is in press at Psychiatric Times] but already it has made the rounds of the blogs and is being read and debated widely. Now, the APA has just released this rather stunning response…
In his editorial, Dr. Frances criticizes the evolving DSM-V on multiple levels, and makes the following claims:• The process of writing the manual is less transparent and less inclusive than the process he oversaw when he chaired the DSM-IV committee.• The underlying science of psychiatry has not advanced enough to merit the kind of extreme makeover proposed by the DSM-V chairpeople:
"The simple truth is that descriptive psychiatric diagnosis does not need and cannot support a paradigm shift. There can be no dramatic improvements in psychiatric diagnosis until we make a fundamental leap in our understanding of what causes mental disorders. The incredible recent advances in neuroscience, molecular biology, and brain imaging that have taught us so much about normal brain functioning are still not relevant to the clinical practicalities of everyday psychiatric diagnosis. The clearest evidence supporting this disappointing fact is that not even one biological test is ready for inclusion in the criteria sets for DSM-5."• The main change being proposed—the official inclusion of a series of rating scales into the diagnostic criteria—is poorly conceived because busy clinicians will reject this extra paper-work.• Other proposed changes in DSM-V will make it too easy to over-diagnose a range of conditions:
“The result would be a wholesale imperial medicalization of normality that will trivialize mental disorder and lead to a deluge of unneeded medication treatment–a bonanza for the pharmaceutical industry but at a huge cost to the new false positive "patients" caught in the excessively wide DSM-V net. They will pay a high price in side effects, dollars, and stigma, not to mentions the unpredictable impact on insurability, disability, and forensics.”Frances’ article is compelling, not only because of the substance of his arguments but because of his clear and forceful writing style. With each sentence, you get a sense that this man has carefully thought through all of these issues and is passionately concerned about the future of his field.
“Both Dr. Frances and Dr. Spitzer have more than a personal “pride of authorship” interest in preserving the DSM-IV and its related case book and study products. Both continue to receive royalties on DSM-IV associated products. The fact that Dr. Frances was informed at the APA Annual Meeting last month that subsequent editions of his DSM-IV associated products would cease when the new edition is finalized, should be considered when evaluating his critique and its timing.”
"It is, however, completely unclear that his lack of enthusiasm is based on any scientifically rigorous foundation. Indeed, his knowledge of these methods seems lacking. Finally, Carroll is quick to point out the acknowledged potential conflicts of others as if they have led to bias in reporting of scientific information. In this case, it is Carroll who has the overwhelming conflict of interest. As developer, owner, and marketer of the Carroll Depression Scale–Revised, a traditional fixed-length test, it is not surprising that the paradigm shift described in our article would be of serious concern to him."
In our military, we have an article, Article 113: Conduct Unbecoming an Officer and a Gentleman [shortened now to Conduct Unbecoming an Officer]. Uncharacteristically, considering the usual military penchant for details, it’s very loosely defined. We don’t need a definition, because we all know what it means. It refers to the fact that we hold our leaders to a higher standard of conduct than the simple standards of the civil and criminal laws. There’s a similar implicit standard in Medicine.
by Kupfer DJ.Biological Psychiatry. 1976 Apr;11(2):159-174.
Previous investigations have indicated that one of the most consistent EEG sleep findings in depressive patients has been a shortened REM latency. On the basis of these studies, we have concluded that with the exception of drug withdrawal states [such as CNS depressant or amphetamine withdrawal and narcolepsy] shortened REM latency points to a strong affective component in the patient’s illness. Short REM latency has also been observed in patients suffering from schizo-affective illness as well as in certain schizophrenic patients who require tricyclic antidepressants in their management. Furthermore, this psychobiologic marker is a persistent, rather than a transient phenomenon, and can be observed over a period of several weeks unless a patient’s condition becomes more favorable through clinical intervention. This present report indicates that short REM latency is found in virtually all primary depressive illness and is absent in secondary depression. Thus, REM latency appears to be a dependable, measurable marker for diagnosing primary depression, and we argue that the phenomenon is independent of age, drug effect and changes in other sleep parameters. It is expected that EEG sleep and motor measurements can yield further significant data and improve differential diagnosis in psychiatry, in much the same way that laboratory data support other medical specialities.
But this was a different Dr. Kupfer on a different trajectory. Then recently, we happened on to another story [see insider trading…]. This Dr. Kupfer was a coauthor and business partner with statistician Robert Gibbons in developing a computerized screening instrument for depression and anxiety. It was developed using NIMH grants by Dr. Gibbons. It was discussed in a series of papers in our best journals. But it was not mentioned as a conflict of interest by any of the authors even though it turned out to be a mature commercial enterprise waiting to launch. Only after it was exposed did the authors offer an apology for failing to list it as a COI. but offered no explanation. To make things remarkably worse, this screening test aims to capitalize on a part of the DSM-5 present from inception – Dimensional Diagnosis [as in anxiety and depression] – a part of the DSM-5 specifically championed by Dr. Kupfer from the outset. This isn’t just the appearance of a COI. This is a COI. This is the kind of thing you can go to prison for in the dog-eat-dog business world. And Medicine should and does have a higher standard than that.
Lest you think this connection between Dr. Kupfer and Gibbons company and the DSM-5 is just circumstantial evidence, try this on for size. Today, I was pointed to something else. This is from Dr. Jane Costello‘s letter of resignation from the DSM-5 Task Force in 2009 [reproduced here from the Carlat blog]:
The tipping point for me was the memo from David and Darrell on February 18, 2009, stating “Thus, we have decided that one if not the major difference between DSM-IV and DSM-V will be the more prominent use of dimensional measures in DSM-V”, and going on to introduce an Instrument Assessment Study Group that will advise workgroups on the choice of old scale measures or the creation of new ones. Setting aside the question of who “decided”, on what grounds, anyone with any experience of instrument development knows that what they proposed last month is a huge task, and a very expensive one. The possibility of doing a psychometrically careful and responsible job given the time and resources available is remote, while to do anything less is irresponsible…hat tip to Uri…
And what about "the memo from David and Darrell on February 18, 2009, stating ‘Thus, we have decided that one if not the major difference between DSM-IV and DSM-V will be the more prominent use of dimensional measures in DSM-V’, and going on to introduce an Instrument Assessment Study Group that will advise workgroups on the choice of old scale measures or the creation of new ones." I presume from this that in 2009, they still fantacized adding Dimensions to the DSM-5, quantified by psychometrics. That was certainly apparent in their symposium, Dimensional Aspects of Psychiatric Diagnosis, in 2006.
Something very unusual happened in the process of the revision of the psychiatric diagnostic manual – the DSM-5 revision published last May. The leaders of the previous revisions, Robert Spitzer [DSM-III, DSM-IIIR] and Allen Frances [DSM-IV], both became outspoken critics of the enterprise and went public with their dissatisfaction. Dr. Spitzer was refused access the the minutes of the DSM-V meetings, and then found out that all the members of the Task Force had signed confidentiality agreements. In a series of articles in the Psychiatric News in 2008 and early 2009, he repeatedly pointed out that secrecy was unprecedented and incompatible with the charge of the Task Force. Dr. Allen Frances declined to join Dr. Spitzer’s campaign until May when he learned about some of the things the DSM-5 Task Force were contemplating, and he went public with his concerns with what became the very public campaign that continues to the present [see dangerous men…]. The response from the APA was silence, defensiveness, or attacks – but never engagement.
Need to Explore the Possibility of Fundamental Changes in the Neo-Kraepelinian Diagnostic Paradigm… Disorders in DSM-III were identified in terms of syndromes, symptoms that are observed in clinical populations to covary together in individuals. It was presumed that, as in general medicine, the phenomenon of symptom covariation could be explained by a common underlying etiology. As described by Robins and Guze [1970], the validity of these identified syndromes could be incrementally improved through increasingly precise clinical description, laboratory studies, delimitation of disorders, follow-up studies of outcome, and family studies. Once fully validated, these syndromes would form the basis for the identification of standard, etiologically homogeneous groups that would respond to specific treatments uniformly.
In the more than 30 years since the introduction of the Feighner criteria by Robins and Guze, which eventually led to DSM-III, the goal of validating these syndromes and discovering common etiologies has remained elusive. Despite many proposed candidates, not one laboratory marker has been found to be specific in identifying any of the DSM-defined syndromes. Epidemiologic and clinical studies have shown extremely high rates of comorbidities among the disorders, undermining the hypothesis that the syndromes represent distinct etiologies. Furthermore, epidemiologic studies have shown a high degree of short-term diagnostic instability for many disorders. With regard to treatment, lack of treatment specificity is the rule rather than the exception.
The efficacy of many psychotropic medications cuts across the DSM-defined categories. For example, the selective serotonin reuptake inhibitors [SSRIs] have been demonstrated to be efficacious in a wide variety of disorders, described in many different sections of DSM, including major depressive disorder, panic disorder, obsessive-compulsive disorder, dysthymic disorder, bulimia nervosa, social anxiety disorder, posttraumatic stress disorder, generalized anxiety disorder, hypochondriasis, body dysmorphic disorder, and borderline personality disorder. Results of twin studies have also contradicted the DSM assumption that separate syndromes have a different underlying genetic basis. For example, twin studies have shown that generalized anxiety disorder and major depressive disorder may share genetic risk factors [Kendler 1996]…
For most of the DSM-III disorders, however, the etiology is unknown. A variety of theories have been advanced, buttressed by evidence – not always convincing – to explain how these disorders came about. The approach taken in DSM-III is atheoretical with regard to etiology or pathophysiological process except for those disorders for which this is well established and therefore included in the definition of the disorder. Undoubtedly, with time, some of the disorders of unknown etiology will be found to have specific biological etiologies, others to have specific psychological causes, and still others to result mainly from a particular interplay of psychological, social, and biological factors. The major justification for the generally atheoretical approach taken in DSM-III with regard to etiology is that the inclusion of etiological theories would be an obstacle to use of the manual by clinicians of varying theoretical orientations, since it would not be possible to present all reasonable etiologic theories for each disorder.Robert Spitzer, in the DSM-III, p 6.
by David J. Kupfer, M.D. and Darrel A. Regier, M.D., M.P.H.American Journal of Psychiatry 168:672-674, 2011.
In the initial stages of development of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders,we expected that some of the limitations of the current psychiatric diagnostic criteria and taxonomy would be mitigated by the integration of validators derived from scientific advances in the last few decades. Throughout the last 25 years of psychiatric research, findings from genetics, neuroimaging, cognitive science, and pathophysiology have yielded important insights into diagnosis and treatment approaches for some debilitating mental disorders, including depression, schizophrenia, and bipolar disorder. In A Research Agenda for the DSM-V, we anticipated that these emerging diagnostic and treatment advances would impact the diagnosis and classification of mental disorders faster than what has actually occurred…
Medscape Newsby Jeffrey A. Lieberman, MD09/28/2011
…we anticipated that this iteration of the DSM would incorporate biological markers and laboratory-based test results to augment the historical and phenomenologic criteria that traditionally are used to establish psychiatric diagnoses. Sadly, this has proved to be beyond the reach of the current level of evidence…
 |
|
Attention Site Visitors
**Effective 12/31/13** UBM Canon has ceased the production of the following brands: Med Ad News, PharmaLive.com, Pharmalot, eKnowledgeBase and R&D Directions. Thank you for your loyalty and support that has played such an important part over the last thirty years. We invite you to visit MedTech World or UBM Canon for additional industry news and media resources. Please contact our customer service group with questions at PLsubs@sunbeltfs.com.
|
But Ed Silverman lives on!Ed Silverman can still be reached by writing to pharmalot@gmail.com
Don’t it always seem to goThat you don’t know what you got ’til it’s gone.They paved paradise,Put up a parking lot.
by Erich LindemannAmerican Journal of Psychiatry. 1944 101:141-148.