The Ghosts in the Machine
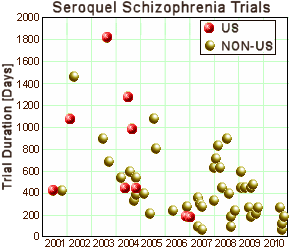 The Clinical Research Organizations are largely invisible. Their main advertised function is to set-up and conduct clinical trials quickly [and successfully]. The graph on the right is from www.clinicaltrials.gov and shows the time from submission to completion of all of Astrazeneca‘s Seroquel clinical trials in Schizophrenia. It demonstrates Parexel‘s success in shortening trial duration [and it shows "globalization" – mostly to Eastern Europe]. I thought it would be worth looking at the web sites of the top CROs to see how they bring off streamlining the process, but they’re written too vaguely to nail down, so I’ll only mention a few notables:
The Clinical Research Organizations are largely invisible. Their main advertised function is to set-up and conduct clinical trials quickly [and successfully]. The graph on the right is from www.clinicaltrials.gov and shows the time from submission to completion of all of Astrazeneca‘s Seroquel clinical trials in Schizophrenia. It demonstrates Parexel‘s success in shortening trial duration [and it shows "globalization" – mostly to Eastern Europe]. I thought it would be worth looking at the web sites of the top CROs to see how they bring off streamlining the process, but they’re written too vaguely to nail down, so I’ll only mention a few notables:
-
Mining Medical Records: When I was looking at the policies promoted by the industry professional organization [ACRO] in the last post, there were a couple I didn’t understand:• Ensure ease of access to data necessary to conduct research while considering patient privacy• Encourage health information technology policies that include consideration of medical research needsThey use data-mining of medical records to locate subjects [when they can] and would like to do a lot more of that in their recruiting.
-
Prescreening: Several CROs mention having large databases of pre-screened subjects available to access when the right study comes along.
-
Globalization: They all mention some version of "emerging" or "non-traditional" countries. A quote:Different therapeutic indications tend to cluster in different regions of the world. Quintiles staff in non-traditional regions includes physician specialists in key therapeutic areas to help fine tune study design and protocols. Patients in non-traditional regions have a high rate of protocol compliance and low dropout rates, because of the high standards of medical care available through clinical trials. This and our combination of global reach and local knowledge can result in speedy recruitment of specific patient populations.Overseas clinical trials are a big deal with each of the large CROs.
-
Communications: Xcellerate® [Covance]; Trial Enrollment Accelerator [Quintilles]; Internet, email, social media, call centers, etc. They have it all. And it’s ongoing, not study specific so they can begin advertising and enrolling for a new clinical trial the day the get the contract. Most of them use sophisticated software to locate high yield sites for a given study.
Ghostwriters and the Demigods:
| While I doubt the term Demigods will achieve general usage to describe the Physicians who allow the Pharmaceutical Companies to use their names as authors in the medical literature to publish these quaesi-scientific "experimercials," it works for me. Demigods [AKA Titans] comes from the Tibetan Wheel of Life, and describes people who aspire to be Gods, but have only made it part-way. In this incarnation, they pad their resumes with publications, jump on each new break-through treatment, and are vulnerable to engagement on the illusionary stage as key opinion leaders, briefly experiencing the adoration they envy but can’t quite attain on their own. The Pharmaceutical Industry has had a field day capitalizing on their unfullfilled aspirations. Like all personalities driven by envy, they become quite defensive and "prickly" when confronted with their deceit. It appears they actually believe their own mythology and the compliments of their handlers. |  |
The POGO examples are of Demigod/Ghostwiter pairings from Scientific Therapeutic Information, a medical writing company, not specifically a CRO. But in the unsealed documents and articles in the Seroquel story, there are a number of instances that suggest Demigod/Ghostwiter‘s are at work in the Halls of the CROs as well. All of the publications of the Seroquel trials have the same format: a lead academic author, multiple Zeneca/AstraZeneca authors, and somebody who probably did the writing in the acknowledgements. In the early preapproval articles, it was Suzanne Bristow-Marcalus, a pharmacy-type who either worked at or for Zeneca. After 2002 when AstraZeneca signed on Parexel, the probable writers were Parexel employees, Aruna Seth and Max Brady – recall the particularly incriminating episode with Dr. Nassir Ghaemi [selling seroquel VII: indication sprawl…]. Later articles appear to have another medical writing company involved [Extended Release Quetiapine Fumarate Monotherapy for Major Depressive Disorder: Results of a Double-Blind, Randomized, Placebo-Controlled Study]. They acknowledge Jocelyn Woodcock of Complete Medical Communications in that article. It most cases, we have to infer Demigod/Ghostwiter pairings from the acknowledgements. They are difficult to prove because they don’t say Demigod: Dr. Richard Borison, Ghostwriter: Suzanne Bristow-Marcalus for obvious reasons.
I suspect that in practice, the CROs that actually do the Clinical Trials are heavily involved in writing up the Approval Documents for the FDA. Likewise, I expect that the published articles that make it into our literature are often written by the CROs that do the studies in conjunction with the Pharmaceutical Companies. The role of the Demigods is to legitimize the articles to Journal editors and general readers. Of course they don’t advertise such activities on their web-sites. Here are representative pages for what they do say about their Medical Writing departments:
| Medical Writing
|
|
| Quintiles | Icon |
| Pharmaceutical Product Development | Kendle |
| Covance | PharmaNet Development Group |
| Charles River Laboratories | PRA International |
| Parexel | INC Research |
It’s equally frustrating trying to run down who manages the recruitment, scheduling, communications with, and presentations by, the Key Opinion Leaders – the Physicians who ride the circuit giving talks at meetings, dinners, and CME presentations that are essentially stealth drug advertisements. This slide show from Quintiles is an example of the involvement of the CROs with the KOL networks [Improved Commercialization Strategy through Stakeholder Network Mapping]. Here’s another approach from Parexel [Reach KOLs On-Line]. My impression is that the KOLs are connected with the Pharmaceutical Companies, but that the CROs are heavily involved in creating presentations, and maintaining communication networks. I can’t penetrate further [because they don’t want us to know how all this works].
Practical Ghostbusting for Rookies

Results of a Double-Blind, Randomized, Placebo-Controlled Study
by Richard Weisler, J. Mark Joyce, Lora McGill, Arthur Lazarus, Johan Szamosi, and Hans Eriksson

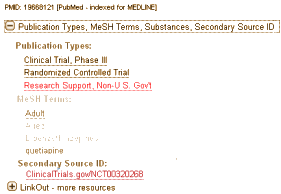
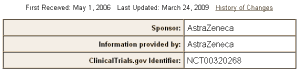
The Clinical Trial – A Ghost of its Former Self
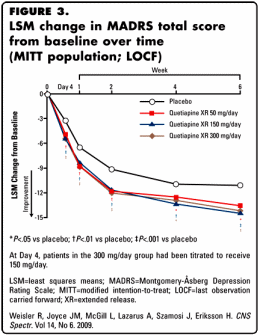 I criticized this article earlier as an example of a CRO-Chart article that may [or may not] have achieved significance, but was irrelevant, based on the reported data [selling seroquel III: the data factories…]. Here, I am suggesting more – that this article in the medical literature is little more than a stealth ad, a way to advertise Seroquel XR [extended release and patent extending] as an off-label first-line treatment for Major Depressive Disorder. The article reeks of bias and deliberate obfuscation of the primary data. AstraZeneca did not get Seroquel XR approved as monotherapy in MDD, but not because of the likely bias and inadequacy of this study. They were turned down because the FDA saw the side effects as too dangerous to approve it for this indication as a primary treatment.
I criticized this article earlier as an example of a CRO-Chart article that may [or may not] have achieved significance, but was irrelevant, based on the reported data [selling seroquel III: the data factories…]. Here, I am suggesting more – that this article in the medical literature is little more than a stealth ad, a way to advertise Seroquel XR [extended release and patent extending] as an off-label first-line treatment for Major Depressive Disorder. The article reeks of bias and deliberate obfuscation of the primary data. AstraZeneca did not get Seroquel XR approved as monotherapy in MDD, but not because of the likely bias and inadequacy of this study. They were turned down because the FDA saw the side effects as too dangerous to approve it for this indication as a primary treatment.
Texas and 37 States Reach $68.5 Million Settlement With AstraZeneca
Attorney General of Texas
Settlement comes after three-year investigation into marketing of Seroquel
March 11, 2011AUSTIN – Texas Attorney General Greg Abbott and 37 other state attorneys general today reached a record-breaking agreement with AstraZeneca, which the states investigated for improper marketing practices. The states’ $68.5 million settlement resolves a three-year investigation into AstraZeneca Pharmaceutical’s efforts to market the antipsychotic drug Seroquel. Under today’s settlement, the State of Texas will receive $3.8 million. Today’s multistate agreement stems from a complaint in federal court charging AstraZeneca with unlawfully marketing Seroquel for unapproved – or off-label – uses. The states also charged AstraZeneca with failing to disclose Seroquel’s harmful side effects and concealing scientific data that revealed safety concerns…
Under the settlement, AstraZeneca agreed to comply with state and federal laws governing its marketing – including legal requirements that prohibit manufacturers from promoting their products for off-label uses not approved by the U.S. Food and Drug Administration [FDA]… The states’ legal action explained that AstraZeneca targeted Alzheimer’s patients at nursing homes, as well as patients who suffered anxiety, depression, sleep disorders and post traumatic stress syndrome. The settlement also imposes injunctive provisions that require AstraZeneca to:
• Publish any payments it makes to physicians on the Internet;• Implement policies that ensure its marketing and sales personnel are not financially compensated for marketing off-label uses;• Establish polices to ensure that its sales personnel will refrain from marketing Seroquel to health care providers who are unlikely to prescribe it for an FDA-approved use; and• Cite Seroquell’s FDA-approved indicators when referencing selected symptoms.Atypical antipsychotics, including Seroquel, can produce dangerous side effects, including weight gain, hyperglycemia, diabetes, cardiovascular complications, an increased risk of mortality in elderly patients with dementia and other severe conditions.
You should have been on the FDA approval panel discussion committee, clearly they did not have anyone with such common sense and ability to see plain sight reason not to (keep) approve Seroquel, again and again —imagine why FDA approved use for this drug in kids age 10!??
Same day as the multi-state litigation settlement for AZ came through the news, my anonymous source received a letter from attorney stating the diabetes (off-label use) settlement packet will arrive in approx 3 weeks. My source, and victim of THE CRIME OF ILLEGAL MARKETING OF A DRUG BY ASTRAZENECA, has diabetes, sued AstraZeneca and will (rumor and Bloomberg says) has it that the victims will only receive $10-12K and that is BEFORE taxes and attorney fee reduction. For a lifetime body damage of an organ that now shortened the victims lives by 5-6 years, all because they were offered Seroquel and most off-label for insomnia not approved for that use and promoted to doctors via reps by AstraZeneca!!
AstraZeneca’s mouthpiece Tony Jewell states AstraZeneca “denies allegations”.
Look at the documents in this series on this blog and then sit back and wonder who has been duped and innocent people continue to be injured/killed by this antipsychotic being promoted now as an antidepressant!
The irony increases when you see that Richard “Smoke and Mirriors” Lawrence now works for a medical writing company!
http://www.bnet.com/blog/drug-business/astrazeneca-8217s-8220smoke-and-mirrors-8221-man-has-new-job-in-medical-writing/1111
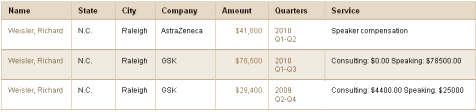
Richard Weisler is currently pocketing income from:
http://projects.propublica.org/docdollars/search?term=Richard+Weisler&state%5Bid%5D=
in ProPublica Dollars for Docs database
$41,800-AstraZeneca
$78,500-GSK
$29,400-GSK
for “Speaking and consulting”
oh oops, did not see it in the post! sorry for double exposure of the doctor in dollars for docs!
Yeah. Weisler has cut quite a swath as a KOL. One wonders what he does for UNC and Duke that justifies his using his adjunct affiliations as credentials.
Hey Mickey, have you seen the movie “Inside Job” (Academy Award winner for Best Documentary 2010)?
Saw it last night, and couldn’t help but think about this series of articles. In fact, I think someone should take the script of that movie, take out “CDOs” and “credit default swaps” and replace them with “atypical antipsychotics” and “SSRIs”; replace the banking execs with KOLs; and replace Lehman Brothers and Goldman Sachs with Emory and Stanford, and you’d have a fairly accurate story.
Steve,
That analogy hits me at times too – it even fits temporally. Something happened that infected the land in multiple places. I’ll put Inside Job on the must-see list…