This post is a continuation of the last one [
significant I…]
using the same reference articles [A1-A5, B1-B3, & C1-C2]
…
The question of suicidality as an adverse effect from SSRIs in adolescents and children is no small matter. I don’t think anyone is saying
never use these drugs in youth [at least I’m not]. The point is to be vigilant and to warn parents and care-takers to keep a close eye on kids on antidepressants if the risk/benefit ratio justifies their use in a given case [at least that’s what I think]. When the black box warning was added in 2004, prescriptions plummeted. While that might be bad for drug sales, that’s not the business of physicians. But the issue keeps coming back up. In
Gibbons’ recent article {
B1} he says in the Abstract…
For youths, no significant effects of treatment on suicidal thoughts and behavior were found, although depression responded to treatment. No evidence of increased suicide risk was observed in youths receiving active medication.
… a conclusion mirroring his findings in a host of his earlier publications [in
a book review… {
B2}]. His analysis included the TADS trial we looked at in the last post [
significant I…]. Furthermore, that TADS trial article [
Suicidal Events in the Treatment for Adolescents with Depression Study (TADS) {
A5}] specifically reported no findings consistent with the akathisia emphasized by Dr. Healy in his writings on this topic. And the problem is that articles like these two {
A5,
B1} don’t disappear from the literature. They just sit there for time immemorial waiting to be found by someone looking for guidance. That’s what the medical literature is for.
As you might expect, both David Healy {C1} and Robert Whitaker {C2} jumped on Robert Gibbons‘ article {B1} soon after it came out [me too {B2}]. Both authors endorsed the findings of Göran Högberg laid out in significant I… – each with a version of his table:
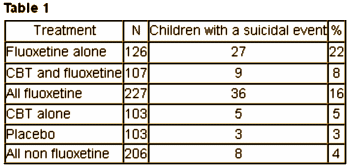
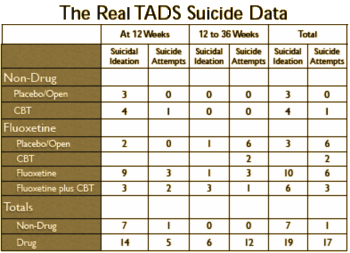
Whitaker {
C2} has a more detailed travelog through the TADS study showing how the numbers were jury-rigged in all the studies.
Healy {
C1} addresses the fact that there were some other studies besides TADS in Gibbon’s meta-analysis:
There were two other pediatric suicide trials of Prozac used by Gibbons and colleagues in this paper. These employed maneuvers 7 and 8 from the less commonly listed strategies table in my post The tricks that drug companies do live after them. That is, patients responding to placebo were dropped from one study, and patients doing poorly on Prozac were dropped from another. Despite these steps, there was still an excess of suicidal acts on Prozac. Dr Gibbons and colleagues apparently “found no evidence that fluoxetine increased the risk of suicidal thoughts or behavior in youths.”
and takes us on a tour of Gibbons’ articles. I’ve just listed some high points from each of their articles. They both speak eloquently for themselves and have a lot more to say about this. I’d suggest a full reading of each by anyone who cares about this topic at all {
C1,
C2}.
I left some room at the bottom for an opinion of my own. The The Treatment for Adolescents With Depression Study (TADS) was a National Institute of Mental Health funded trial, the kind we’re supposed to trust. If you read all the articles, the actual suicidality information is buried in layers of numerology, hidden from view until an astute Swedish reader found the real results hidden in plain view [to borrow Dr. Healy’s term]. 82% of the suicidal events were in kids on Prozac. 94% of the suicide attempts were by kids on Prozac. You can’t miss something like that by accident [another of Dr. Healy’s phrases was eyes wide shut]. Dr. Gibbons papers started with the idea that the black box warning caused an increase in the suicide rate {B1} [thoroughly debunked] and have now moved to this offering, saying there was no cause for the warning in the first place {B2}. His paper is a tangle of statistical analyses no reader of the Achives of General Psychiatry could possibly follow or understand [and even if they could, the data wasn’t included]. That also doesn’t seem like an accident to me.
And Dr. Healy, who has been talking about this for years [once losing a job over it at the hands of the now infamous Dr. Nemeroff], isn’t saying not to use the SSRIs in adolescents ever. He said this a long time ago {
C1}:
As I also mentioned in the 1991 letter to the BMJ: “The significance of the emergence of suicidality.. is that it can be anticipated and forestalled by warning patients.” I once thought that an appeal to patient safety would get doctors on board – but apparently not.
He’s saying that we need to be careful, not prescribe these drugs casually in adolescents, and if we do use them to be vigilant for the emergence of suicidality. I say that too, having seen it happen {
B1}.
This is deliberately deceitful science published in our major medical journals funded by our government. And the people calling it to task for what it is are a Swedish PhD, a Welsh Psychiatrist, and an independent journalist/author turned activist. We are forever in their debt for doing it, but it’s pretty pitiful that they’re the ones that have to step up to the plate. The authors of these journal articles represent our major medical schools, are funded by our highest agencies of medicine, have had their work vetted by journal editors and reviewed by peers, and those articles still landed in our literature for time immemorial waiting to be found by someone looking for guidance. And they’re bullshit – dangerous bullshit at that. I can’t think of any other way to say it.
That’s significant!…


Thank goodness for this excellent site. Once again, a sound, thorough common sense analysis of what data does and does not show, and why Dr Gibbons and his Pharma-funded ilk are so dangerous. I wish I had found information like this, and the information in Dr Healy’s books, before my son died by suicide after taking a drug he did not need. There are too many doctors who are not only casual, but actively dismissive of parental concerns and questions. I only wish there was a way to beam this knowledge out to the thousands or millions of parents who are being misled and are trusting in professionals who have either been bought or are not paying attention.
I would be standing up giving you hearty applause after hearing you read that last paragraph in front of an audience of your PEERS!
Thank you Doctor!
This direct TADS NIMH data MUST be made VERY available available and well known to the scientific community. Decisions are made DAILY throughout the Western world to place children on fluoxetine and other SSRI’s as a direct result of the intitial fraudulent data. Child psychiatrists cannot know of the real data without such HIGH LEVEL PUBLICATION of these incredibly important findings.
With the greatest gratitude for your work.
John S March failures to disclose:
2006 paper: The Treatment for Adolescents With Depression Study (TADS): Methods and Message at 12 Weeks. JAACAP 2006 March, Silva, Vitiello, TADS team – Correspondence to Dr. John March – Disclosure: Dr. March is a consultant or scientific advisor to Pfizer, Eli Lilly, Wyeth, GlaxoSmithKline, Jazz, and MedAvante; he is a stockholder in MedAvante; he is on the speakers’ bureaus of Pfizer and Eli Lilly; and he receives research support from Eli Lilly, Pfizer, and Wyeth. Dr. Silva is a consultant for Pfizer. Dr. Vitiello has no financial relationships to disclose.
2007 paper: The Treatment for Adolescents With Depression Study (TADS)
Long-term Effectiveness and Safety Outcomes. Arch Gen Psych, 2007 TADS team. Correspondence: John S. March, MD. Submitted for Publication: August 28, 2006; final revision received December 23, 2006; accepted December 26, 2006.
Financial Disclosure: Dr Findling has received research support, acted as a consultant, and/or served on a speaker’s bureau for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Celltech-Medeva, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Johnson & Johnson, New River, Novartis, Otsuka, Pfizer, Sanofi-Aventis, Shire Pharmaceuticals, Solvay, and Wyeth Research. Dr Posner has received funding from the US Food and Drug Administration (FDA) to develop the suicidality classification system used in their antidepressant safety analysis. Subsequently, as part of an effort to help execute the FDA suicidality classification mandates, Dr Posner has had research support from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Eisai Inc, Forest Laboratories, GlaxoSmithKline, Johnson & Johnson, Novartis, Organon USA, Sanofi-Aventis, Shire Pharmaceuticals, UCB Pharma, and Wyeth Research.
Funding/Support: The TADS is supported by contract RFP-NIH-NIMH 98-DS-0008 from the NIMH to Duke University Medical Center (principal investigator, John S. March, MD, MPH). Eli Lilly and Company provided fluoxetine and matching placebo under an independent educational grant to Duke University.
Disclaimer: The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the NIMH, the National Institutes of Health, or the Department of Health and Human Services. Eli Lilly and Company had no role in the design or implementation of the study, data analysis, or in the writing of this manuscript.
AT THE TIME OF SUBMISSION, MARCH WAS A CONSULTANT OR ADVISOR TO, AND ON THE SPEAKERS BUREAU FOR LILLY. AND DID NOT DISCLOSE.
He has similarly lied in 2012 paper Drug Development in Pediatric Psychiatry: Current Status, Future Trends. Child and Adolescent Psychiatry and Mental Health 2012 in stating – Dr. March has not engaged in industry promotional work, e.g., speakers bureau or training, for over 15 years. Some simple maths – that’s a lie.
Dr Marsh remains co-chair of NAMHC’s Neurodevelopment Workgroup Roster – a key advisory body to the NIMH – and must surely be removed forthwith for this egregious dishonesty, and his publications where his enormous undisclosed COI have occurred, eg TADS, should also surely be retracted from the literature.
John S March COI breaches continued:
2006 paper: The Treatment for Adolescents With Depression Study (TADS): Methods and Message at 12 Weeks. JAACAP 2006 March, Silva, Vitiello, TADS team – Correspondence to Dr. John March – Disclosure: Dr. March is a consultant or scientific advisor to Pfizer, Eli Lilly, Wyeth, GlaxoSmithKline, Jazz, and MedAvante; he is a stockholder in MedAvante; he is on the speakers’ bureaus of Pfizer and Eli Lilly; and he receives research support from Eli Lilly, Pfizer, and Wyeth
Critically important, oft quoted / referenced 2007 Archive General Psychiatry paper:
The Treatment for Adolescents With Depression Study (TADS)
Long-term Effectiveness and Safety Outcomes. Arch Gen Psych, 2007 TADS team. Correspondence: John S. March, MD. Submitted for Publication: August 28, 2006; final revision received December 23, 2006; accepted December 26, 2006. – NO DISCLOSURES WHATSOEVER FROM JOHN MARSH
2009 Journal Clinical Psychiatry paper Suicidal Events in the Treatment for Adolescents with Depression Study (TADS) John March is a consultant or scientific advisor to Pfizer, Lilly, Wyeth, GSK, Jazz, and MedAvante and holds stock in MedAvante; he receives research support from Lilly and study drug for an NIMH-funded study from Lilly and Pfizer.
KEY NIMH POSITIONS:
NAMHC’s Neurodevelopment Workgroup Roster
http://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/namhc-workgroups/namhcs-neurodevelopment-workgroup-roster.shtml
AND A REPORT http://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/neurodevelopment_workgroup_report.pdf
Scandalous. Deserving of high profile publicity and retraction of positions and papers.
Okay, so my theory of anosognosia was wrong. This is sheer evil, or as close to it as academic intellectual dishonesty can get.
Dr. Mickey, have you thought of publishing on Robert Whitaker’s blog, too? He’s quite accessible and is taking on other contributors.