While the Treatment for Adolescents With Depression Study (TADS) involved only Prozac as a study drug, there were plenty of other companies invested in countering the FDA’s 2004 Warning about suicidality in adolescents on SSRIs:
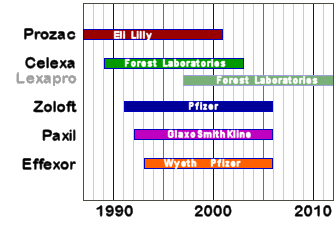
[patent life of the antidepressants]
Now looking at the Financial Disclosures from the four key TADS articles that I mentioned in significant I… and significant II…, these companies were well represented:
-
Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents With Depression
by the Treatment for Adolescents With Depression Study (TADS) Team
JAMA. 2004 292(7):807-820.Financial Disclosures: Dr March has served on the speaker’s bureau for Pfizer and Lilly and has received research support from Lilly, Pfizer, and Wyeth. Dr Findling has received research support from Bristol-Myers Squibb, Forest, GlaxoSmithKline, Lilly, Nature’s Herbs, Organon, Pfizer, Solvay, Somerset, and Wyeth; been a consultant for Bristol-Myers Squibb, Forest, GlaxoSmithKline, Lilly, Pfizer, Somerset, and Wyeth; and served on the speaker’s bureau for Bristol-Myers Squibb, GlaxoSmithKline, Lilly, and Wyeth. Dr Waslick has received research support from Lilly. Dr Walkup has received research support and honoraria from Lilly. Dr Kastelic has received honoraria from Pfizer. Dr Kratochvil has received reseach support from Lilly, Forest, and GlaxoSmithKline; been a consultant for Lilly; and served on the speaker’s bureau for Lilly. Dr Harrington has received research support from Lilly, Pfizer, and Astra-Zeneca. Dr Leventhal has been a consultant, received research support, and served on the speaker’s bureau for Lilly. Dr Emslie has received research support from Lilly, Organon, and RepliGen; been a consultant for Lilly, GlaxoSmithKline, Forest Laboratories, Pfizer, and Wyeth-Ayerst; and served on the speaker’s bureau for McNeil. -
Treatment for Adolescents with Depression Study (TADS): SafetyResults.
by Emslie G, Kratochvil C, Vitiello B, Silva S, Mayes T, McNulty S, Weller E, Waslick B, Casat C, Walkup J, Pathak S, Rohde P, Posner K, March J; Columbia Suicidality Classification Group; TADS Team
Journal of the American Academy of Child and Adolescent Psychiatry. 2006 45(12):1440-55Disclosure: Dr. Emslie receives research support from Eli Lilly, Organon, and Forest Laboratories; is a consultant for Eli Lilly, GlaxoSmithKline, Forest Laboratories, Wyeth-Ayerst, and Pfizer; and is on the speakers’ bureau of McNeil. Dr. Kratochvil is a consultant or scientific advisor to Eli Lilly, Shire, Cephalon, Organon, AstraZeneca, Boehringer-Ingelheim, Abbott and Pfizer; receives research support from Eli Lilly, McNeil, Cephalon, and Abbott; receives study drug for an NIMH-funded study from Lilly; and is on the Eli Lilly speakers’ bureau. Dr. Silva is a consultant to Pfizer. Dr. Weller is a consultant to and receives grants from Otsuka, Johnson & Johnson, AstraZeneca, Organon, Pharma, Shire, and GlaxoSmithKline. Dr. Waslick receives research support from Eli Lilly. Dr. Casat receives research support from Eli Lilly, GlaxoSmithKline, Shire, Bristol-Myers Squibb, AstraZeneca, Sanofi-Synthelabo, Pfizer, and McNeil; is on the Advisory Board and the speakers’ bureaus of Eli Lilly and GlaxoSmithKline. Dr. Walkup receives research support from Eli Lilly, Pfizer, and Abbott; is a consultant to Eli Lilly, Pfizer, Jazz, and Cephalon; and has received honoraria from Eli Lilly and Pfizer. Dr. Pathak receives research support from Forest. Dr. Posner has received research support from GlaxoSmithKline, Forest, Eisai, AstraZeneca, Johnson & Johnson, Abbott, Wyeth, Organon, Bristol-Myers Squibb, Sanofi-Aventis, Cephalon, Novartis, Shire, and UCB Pharma. Dr. March is on the speakers’ bureaus of Pfizer and Eli Lilly and has received research support from Eli Lilly, Pfizer, and Wyeth. The other authors have no financial relationships to disclose. -
The Treatment for Adolescents With Depression Study (TADS)
Long-term Effectiveness and Safety Outcomes
by The TADS Team
Archives of General Psychiatry. 2007 64(10):1132-1143.Financial Disclosure: Dr Findling has received research support, acted as a consultant, and/or served on a speaker’s bureau for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Celltech-Medeva, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Johnson & Johnson, New River, Novartis, Otsuka, Pfizer, Sanofi-Aventis, Shire Pharmaceuticals, Solvay, and Wyeth Research. Dr Posner has received funding from the US Food and Drug Administration (FDA) to develop the suicidality classification system used in their antidepressant safety analysis. Subsequently, as part of an effort to help execute the FDA suicidality classification mandates, Dr Posner has had research support from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Eisai Inc, Forest Laboratories, GlaxoSmithKline, Johnson & Johnson, Novartis, Organon USA, Sanofi-Aventis, Shire Pharmaceuticals, UCB Pharma, and Wyeth Research. -
Suicidal Events in the Treatment for Adolescents with Depression Study (TADS)
by Benedetto Vitiello, Susan Silva, Paul Rohde, Christopher Kratochvil, Betsy Kennard, Mark Reinecke, Taryn Mayes, Kelly Posner, Diane E. May, and John S. March
Journal of Clinical Psychiatry. 2009 70(5): 741–747Financial Disclosure: Susan Silva is a consultant with Pfizer. Christopher Kratochvil receives research support from Eli Lilly, Somerset, Abbott, Shire, McNeil, and Cephalon, is a consultant for Eli Lilly, Pfizer, Cephalon, AstraZeneca, Organon, and Shire, and is on the Speaker’s Bureau of Eli Lilly. Dr. Posner has been consultant and/or received research support from GlaxoSmithKline, Forest, Eisai, AstraZeneca, Johnson&Johnson, Abbott, Wyeth, Organon, Bristol-Myers Squibb, Sanofi-Aventis, Cephalon, Novartis, Shire, and UCB Pharma. John March is a consultant or scientific advisor to Pfizer, Lilly, Wyeth, GSK, Jazz, and MedAvante and holds stock in MedAvante; he receives research support from Lilly and study drug for an NIMH-funded study from Lilly and Pfizer. The other authors have no financial relationships to disclose.
I think we’re so used to this kind of thing that it doesn’t occur to us how really bizarre having a rainbow coalition of Investigators with personal financial ties to pharmaceutical companies with an investment in the outcome of the study actually involved in the study itself. It’s hard to imagine that the connections are unrelated to, for example, publishing this figure in an article titled Suicidal Events in the Treatment for Adolescents with Depression Study…
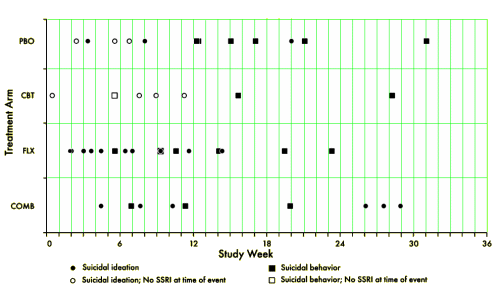
[reformatted for clarity]
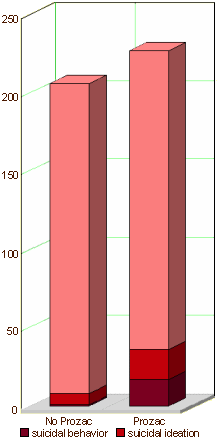 … and failing to mention that 82% of the suicidal events were in kids on Prozac and 94% of the suicide attempts were by kids on Prozac. What other explanation is there besides those conflicts of interest for leaving out the most obvious thing in their data? Where are the graphs in the article that look like these? Raw numbers. One doesn’t even need any statistics to see the point. Like all ‘good data,’ it’s just there.
… and failing to mention that 82% of the suicidal events were in kids on Prozac and 94% of the suicide attempts were by kids on Prozac. What other explanation is there besides those conflicts of interest for leaving out the most obvious thing in their data? Where are the graphs in the article that look like these? Raw numbers. One doesn’t even need any statistics to see the point. Like all ‘good data,’ it’s just there. 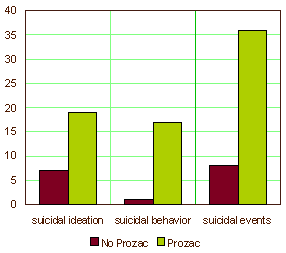
Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents With Depression
by the Treatment for Adolescents With Depression Study (TADS) Team
JAMA. 2004 292(7):807-820. [full text on-line]
This is their outcome graph reformatted by doing that thing I do about making the ordinates start with zero. The absent [B] was another Depression Scale that looked the same as the CDRS-R.
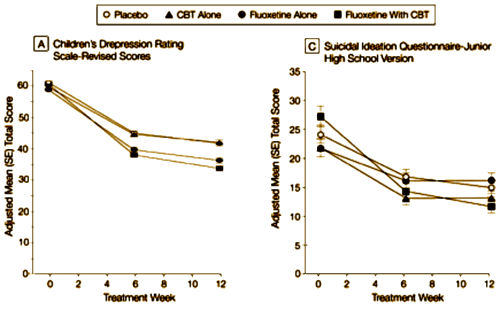
It terms of depression, Prozac helped some. CBT didn’t add much. By this suicidal ideation rating scale, Placebo and Prozac came out close to the same. CBT did seem to decrease suicidal ideation.
The Treatment for Adolescents With Depression Study (TADS)
Long-term Effectiveness and Safety Outcomes
by The TADS Team
Archives of General Psychiatry. 2007 64(10):1132-1143. [full text on-line]
The long-term results only covered the treatment groups [the placebo group was no longer in the study]. The results are harder to interpret because the study was unblinded at 12 weeks and they did all kinds of things thereafter. I will admit to having low libido for trying to make sense of it after about an hour and chose playing with my dogs as a more productive use of time on this gorgeous afternoon. That said, here’s the outcome they reported [adjusted to start at zero]:
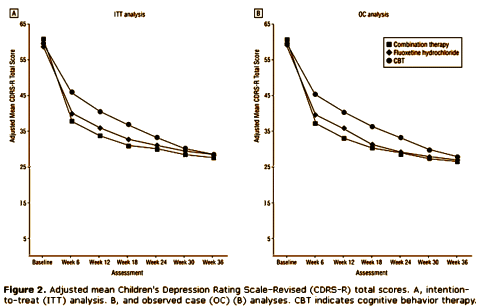
My take by inspection is that one could say that the kids treated with Prozac got better faster, and that depressed kids get better over time anyway [with the reservations that what happened in the second part of the study is not totally clear].
In each of the four papers in the list being looked at from the The Treatment for Adolescents With Depression Study, I had the feeling that there was some level of bias in favor of SSRI effectiveness in adolescent depression and definitely in downplaying emergent suicidality. I’ve tried to show examples in significant I…, significant II…, and this post, but the concrete examples don’t capture it all. For example, I joked around about the difficulty following what happened after the unblinding at 12 weeks, but what I actually felt was that it had been so obfuscated that I’d never figure it out. And there were examples like that in each of the papers. There’s little question that there was a lot more of that when it came to suicidality, the grossest example is illustrated above. If you read paper number 4. [Suicidal Events in the Treatment for Adolescents with Depression Study (TADS)], it seems to have been written to "shoot down" the idea of akathisia as the cause of Prozac’s suicidality. Since there was no actual clinical material but only rating scales, that effort failed from my perspective. But the point is that there’s bias in that orientation, just like there’s BIAS in failing to show the graphs I’ve included above about suicidality.
Did you make all these graphs? I’m impressed. Now just imagine what you could do if you just started taking your medication again and were no longer hampered by that pesky psychosis of yours.
Of course the drug companies are conspiring to take over psychiatry and then the world, but don’t worry, the illuminati will team up with the NWO to stop them!
AllHailXenu,
The dismissive tone, the arrogance…
You must be a psychiatrist, of the heavy script-writing variety, am I right?
The facts are coming out, like never before…
Not conspiracies, just facts.
It must be very painful for you to witness the collapse of your profession.
Not the illuminati, not consipracy nuts…. just the facts.
The data alone will bring down psychiatry.
In fact, it already has.
Duane