Once the data’s in, one is invariably tempted to play with it – find new things, maybe make it look better. There are some conventions to keep data play in bounds. In a hypothesis driven study like Study 329, one is asked to state the hypothesis and to declare the outcome variables that will prove or disprove the it a priori [up-front]. Another temptation is to measure everything under the sun looking for confirmation and report what suits you. As much as we’d like to see the scientist as a detached braniac driven only for the truth, human-ness is just as common in scientists as anyone else. So, in science, there are protocols and conventions in place to hold the science in the road.
The Study 329 authors knew the rules. The a priori protocol was in place, and accurately stated in the text of the article [the red subscripts are mine, for later]:
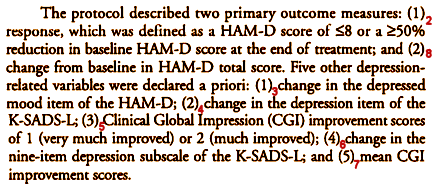
There were two Primary Outcome Parameters. I covered one of them (2) in the first post [the lesson of Study 329: the basics…]. Now, here’s Table 2 from the paper again [the red numbers are mine]:
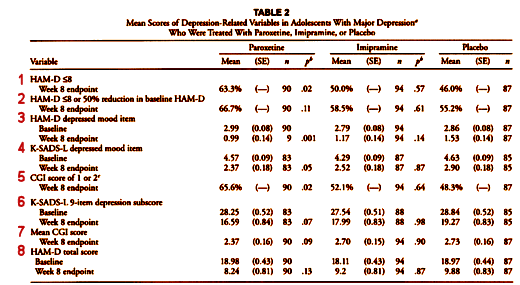
There are several things of note. First, the Primary Outcome variables are not identified in the Table, instead they are shuffled together with the Secondary Outcomes. In fact, the major one is listed at the end, separation of Paroxetine from Placebo [8]. Note that both of the Primary Outcomes were not significant [2 and 8]. The next thing of note is that there were "five other depression related variables" mentioned as secondary outcome measures in the protocol, but there are six others in the table. The extra is number 1. And, by the way, number 1 [HAM-D < 8] is just a partial number 2 [HAM-D < 8 or 50% reduction in baseline HAM-D]! So they’ve essentially ablated the protocol’s distinctions [and thrown in an extra jury-rigged significant parameter]. These are terminal breaks with convention – protocol violations.
It’s not that these things weren’t pointed out along the way as they created this article. This is from Rosemary Oakes, a SKB statistician [03/05/1999] echoed by SKB Exec Jim McCafferty [to Sally Laden]:
and by the JAACAP reviewers:
![]()
"As this was a pharmaceutical-industry sponsered study, it is likely that there was a primary
outcome measure that was identified a priori. If this is the case, the authors should clearly state
what this primary outcome measure was in the METHODS, RESULTS, and COMMENT sections."
And then there was another striking omission, failure to correct for measuring so many parameters [the Bonferroni Correction]. In spite of its funny name, it’s intuitively sensible. If you’re looking at a bunch of parameters the likelihood of finding something by chance increases. So the correction is to up the p value requirement, multiplying by the number of parameters. Here it is mentioned in the JAACAP reviews:
![]()
"Since there was a large number of depression outcome measures used and because a
Bonferroni correction was not employed, this is a particularly important consideration."
And from another feedback letter:
 The response to this last comment [right] was written in as marginalia. It says "<?> suggestion dismissed by
The response to this last comment [right] was written in as marginalia. It says "<?> suggestion dismissed by Keller Ryan <?>" and "since comparisons were a priori – I do not think correction is necessary." The email was sent to Dr. Neal Ryan and it was copied to Dr. Keller so I assume these are their comments At a later time, in a formal response to the JAACAP reviewers addressing this same point, they said:
Both of these responses are laughable – too far off the point to even matter. The much more likely explanation for their reticence is that the Bonferroni Correction would make their table look very differently – one out of eight staying significant:
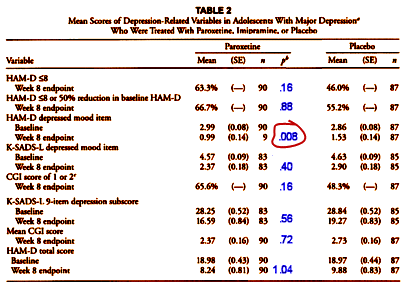
[adapted and modified from the original]
So they embedded the crucial Primary Outcomes in a long list to hide their importance [and non-significance], and then ignored the correction that would be expected for a list this long, again Medical Statistics 101. That’s neither oversight nor sloppiness. That’s deliberate deceit. The only reason for presenting the data like this is to obscure the failure of the study to show a clinically meaningful response to Paroxetine. The reviewers saw it. Even the people at SKB/GSK seemed to see the problems. It was the principal authors that appeared to be fighting to to hold on to the obvious sleight of hand. I was surprised at that. This from SKB/GSK as the paper went to press:





There are more documents available than those available at Healthy Skepticism, namely those released by POGO. They reveal additional information about the communications. I would disagree that the primary problem is that the information flows between them freely. McCafferty appears to be concerned about the way the psychiatric side effects were being presented, and there are strong suggestions that he was interested in making the presentation of them more transparent not more opaque. The documents from POGO, in conjunction with those from Healthy Skepticism, suggests that Ryan and Keller, with Laden, were central to how the information was presented in the final paper. That of the co-authors, the ones who most clearly concurred with Ryan and Keller appear to have been Strober, Wagner, and Birmaher (they all sign off on the response letter to Jureidini). Dulcan appears to have greenlighted the paper without changes suggested by JAACAP reviewers. In the end the 2001 paper exists in the form that it does in JAACAP because of Ryan, Keller, and Dulcan. The editor at JAMA blocked it at that journal. After that no other people in the scientific community (co-authors, those in the Clinical Research department at SKB) were able to divert Ryan and Keller’s approach to what was submitted and revised. And the reviewers’ input to Dulcan did not alter her decision. Reviewing Ryan and Keller’s correspondence the irony is that the 2001 paper may have had a better chance of presenting the information in an even handed fashion because SKB’s clinical research people were involved than had Ryan and Keller just gotten the money from SKB marketing (which they attempt to do in order to circumvent the SKB’s clincal research division). You look at this era as having passed. Again, the documents available suggest that Ryan acted out of belief, NOT out of funding source. This does not suggest that Ryan (and perhaps Wagner who handled the safety data posters) approach research differently today with NIMH funding than they did with SKB/GSK funding. There is a several page document that lays out the relevant POGO documents but the blog does not appear to accept the cut and paste. Please provide a way that it can be posted and I will post it to the comments section. It lays out the relevant information from the POGO archive. There is some relevant information behind AACAP’s paywall but I doubt it would substantively change the interpretation.
These people are the masters and mistresses of deceit and obfuscation in the service of their commercial paymasters. Here is another instance: the outcome you identify as #8 in the Table is NOT one of the prespecified primary outcome measures. The prespecified measure was change from baseline in HAM-D total score. That is not what Table 2 displays. Instead, Table 2 displays the end point HAM-D comparison (LOCF to Week 8). Anyway, it was nonsignificant. The paper actually contains no analyses of the second prespecified endpoint. For cosmetic effect there is a pretty picture of change in HAM-D total score by group over 8 weeks in Figure 2 of the published article, but no analyses! This is the statistical equivalent of hand waving. And these people want to be taken seriously?
Study 329 and the 2001 Paper in the Journal of the American Academy of Child Psychiatry (JAACAP)
“You have not been appointed as the guardian of the journal, or of the profession of child and adolescent psychiatry. Many highly expert child and adolescent psychiatrists were participants in that study, and others that were similar, and others equally expert contributed to the review process. In addition, the prescription of SSRIs for youth, not only in this country but around the world, far predated any research data on their effects with youth, so this article could hardly be praised or blamed for that trend.” – Mina Dulcan
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/Dulcan.pdf
“Science is not run by democracy” – Rachel Klein
http://dida.library.ucsf.edu/tid/hvu38h10
Keller letter to SKB regarding a naturalistic phase of study 329 (1993)
“Enclosed are some items we discussed regarding the naturalistic phase of the unipolar depression study in adolescents. Your interest and enthusiasm to fund this phase of the study is terrific. You and your colleagues at SmithKline Beecham will be part of the world’s largest and most comprehensive database on adolescent depression.” “In the enclosed reprint from the Journal of Affective Disorders my research group discovered underrecognition and undertreatment of adolescents with serious affective illness. Only one adolescent out of 38 received pharmacologic treatment, and the prescribing agent was an anxiolytic.”
“I am confident that the findings generated from this timely research program will result in between 25-40 manuscripts in the leading peer-reviewed journals, such as New England Journal of Medicine. Journal of the American Medical Association. Journal of Affective Disorders. Archives, of General Psychiatry, American Journal of Psychiatry and the Journal of the American Academy of Child and Adolescent Psychiatry. Moreover, every relevant national and international meeting and workshop held during the next decade will ask to have these data presented. Newly written or edited textbooks will have chapters including findings from this research, and all medical students, interns, residents, fellows and health care professionals will have these data as the latest in the field. In the hands of the Principal Investigators running this program, it is a sure bet that this research will become the world’s definitive database on adolescent depression”
“Therefore, the “payoff” to the field of psychiatry and to SmithKline Beecham for the naturalistic add-on to the clinical trial will be enormous for a marginal dollar cost.”
“Thank you very much for your interest and involvement in this exciting enterprise. My Co-Investigators and I look forward to this collaboration with you and SmithKline Beecham.”
“cc: Drs. Geller, Klein, Kutcher, Ryan and Strober”
http://dida.library.ucsf.edu/tid/jou38h10
Ryan and Control Over Which Sites Are Used in 329 re Naturalistic Phase (1993)
“Both Marty and Neil were pleased that we had tentative approval for funding the naturalistic phase and they came prepared to address the issues of the study timings and the pilot study data.”
“3) Additional sites* Both Neil and Marty expressed strong views that they will not accept additional sites. This option had been interrogated by their group and although they identified two additional individuals (Graham Aimsley and Elizabeth Weller ?), neither of these could participate. Elsie asked if the group would like SB to submit some names for their approval. Neil stated that he did not want to do this because if they rejected someone, there may be professional consequences. Elsie told them that prospective investigators would be approached in only the most general of terms without promising participation. Neil still disliked the idea and was wary of others knowing about the study. He reemphasized that limiting the conduct of the study to the present sites has been their strongest view since the beginning”
http://dida.library.ucsf.edu/tid/bou38h10
Status of Coordination (1994)
“Lastly, but probably most importantly, the coordinators indicated that there has been little exchange of information from Dr. Ryan to them. Dr. Birmaher is conducting the necessary medical procedures and the coordinators are doing all of the rest. As I suspect this is the case for the majority of the sites, it is probably essential to have regular communications with the coordinators as well as the investigators.”
http://dida.library.ucsf.edu/tid/qou38h10
Re Keller and Ryan approaching GSK Marketing for Funding (1995)
“Although the rationale for investigating long term outcome has merit, we in Clinical Research have been steadfast in not committing any resources or support to the post treatment follow-up project. We have repeatedly told Drs. Keller and Ryan that our commitment was to the main study only. ) But Keller and Ryan continue to press their case. Keller approached U.S Marketing directly, and according to Keller, U.S. Marketing has agreed to grant funding of $25,000 for 1995, increasing to $75,000 in 1996. The total costs for the full naturalistic follow-up period (which could be operational for the next 5 years) would be between $500,000 and $600,000”
http://dida.library.ucsf.edu/tid/upu38h10
SKB Beebe to Ryan Describing the Meaning of “emotional lability” Label (1998)
SKB McCafferty Cced On This As Well.
“Please note that in the Adverse Experiences Table (14,2.1), under the heading “Nervous System,” the categories Emotional Lability and Euphoria refer to suicidally and mania/hypemania respectively.”
http://dida.library.ucsf.edu/tid/xou38h10
Table of adverse events and assessed relatedness:
http://dida.library.ucsf.edu/tid/aru38h10
Table 5 on page 10; Wagner’s SKB Produced Poster (2000)
http://dida.library.ucsf.edu/tid/dvu38h10
The 1998 Wagner Poster
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/WagnerPoster.pdf
Geller to Ryan (1999)
“i suspect my comment has already received attention, but it is one that I fully believe must be addressed in the abstract because not to do so seems deceptive, notice that this e-mail goes only to you, although we were asked to send to you, Mike and Marty plus Sally. the abstract does not include that IMJ HAD to reach at least 200 mg/day. the sentence on IMI dosage under the paragraph title intervention, must be amended to state that ” was gradually titrated upwards FROM A MINIMUM OF 2000 MG/DAY based on …” will leave this in your capable hands to get inserted, this is a key point because of tne observation that some of the IMI subjects had responded at lower than 200 mg/day and that the IMI toxicity was higher with increasing dose clinically.”
http://dida.library.ucsf.edu/tid/ppu38h10
See related Comment 5 from Reviewer #1 on page 5 (2000)
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/ResponsestoJAMA.pdf
SKB’s McCafferty Expressing Concern to Ryan, Keller, Strober, Linden
Regarding the Reporting of Side Effects (March 1999)
“A reviewer may question the adverse events profile for paroxetine. Although most events reported were similar in nature and frequency to that reported in the adult, there were higher number of paroxetine patients who reported “emotional liability” and “hostility”. The term “emotional liability” was catch all term for “suicidal ideation and gestures”. The hostility term captures behavioral problems, most related to parental and school confrontations. Although the investigators did not feel this was a problems, it may raise a flag if not described.”
http://dida.library.ucsf.edu/tid/uuu38h10
http://dida.library.ucsf.edu/tid/rvu38h10
Klein to McCafferty Regarding Concerns About Reporting of Adverse Events (April 1999)
“Thank you for being so helpful, and sending me the information on adverse effects. I am writing to you because I am confused about some of the data. Specifically, it’s difficult to reconcile me information I received with the data presented at NCDEU, two years ago by Karen Wagner et al. In that paper, she indicated that 10 patients had had “serious adverse events.” I realize the definition of these is different from “adverse experiences leading to withdrawal.” However, it appears that all 10 in Wagner’s poster were withdrawn. Furthermore, 9 are reported as having been hospitalised. Is that accurate? That information is not included in the paper so I’m not sure which report is the accurate one. For clarity’s sake, I attach a copy of the table that was presented at NCDEU. Finally, the paper (Fig.l) indicates that a total of 18 patients withdrew because of protocol violation. What docs that refer to? Similarly, what is the meaning of “lost to follow-up” (N=7) and “other” (N=7) for withdrawal reasons. Moreover, lack of efficacy (N=11) is unclear—did patients leave because they weren’t getting better, or were they dropped by the investigators for this reason? It would be desirable to split withdrawals between patients who opted to drop out, and those that were terminated by the investigators. The information concerning withdrawals is important in terms of understanding the process of conducting systematic studies in this clinical population. Not an easy task. I guess I’m concerned that we not be subject to criticism for presenting inconsistent data.”
http://dida.library.ucsf.edu/tid/lpu38h10
McCafferty Expressing Concern to Linden Regarding the Reporting of Side Effects (July 19th 1999)
“I reviewed the latest version of the manuscript and have some comments. Two are minor but one is of some concern.”
“Major.
Safety. It seems incongruous that we state that paroxetine is safe yet report so many SAEs. I know the investigators have not raised an issue, but I fear that the editors will. I am still not sure how to describe these events. I will again review all the SAEs to make myself feel comfortable about what we report in print.”
http://dida.library.ucsf.edu/tid/tuu38h10
Further Letter to Linden 3 Days Later (July 21st 1999)
“Because the number of patients with serious AEs reported for the paroxetine group (11/93, 12%) is high vis a vis the placebo group (2/87, 2%), additional text may be necessary for prospective. I would provide some definition of an SAE, and also state that the serious events, with few exceptions, were psychiatric in nature(as opposed to physical). We should also describe what events led to hospitalization (n.b. 7 paxil patients not 6). Some suggested text below.”
http://dida.library.ucsf.edu/tid/ssu38h10
GSK Statistician Oakes’ Had Also Raised Concerns Earlier Regarding Endpoints (1998)
http://dida.library.ucsf.edu/tid/mvu38h10
McCafferty May Have Continued to Have Had Concerns (Linden and Keller’s Response) (2000)
“Today, Jim McCafferty asked us to hold this manuscript until the SB legal department can review it one last time.”
http://dida.library.ucsf.edu/tid/bvu38h10
From JAMA Reviewer #2, on page 8 (1999-2000)
“Most importantly, many have assumed that with fewer cardiovascular side effects, TCAs are safer to prescribe. However, given the high rate of primary care prescription of antidepressants and the readership of JAMA, it is important to emphasize that behavioral side effects in a minority of patients treated with paroxetine may be more serious than with TCAs and that they require excellent provision of psychiatric assessment and management, including access to psychiatric hospitalization. In other words, it is easier to assume quality control for ECG administration and reading than to know that all of the primary care physicians prescribing antidepressants have adequate training in monitoring of the psychiatric side effects of SSRIs and other antidepressants.”
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/ResponsestoJAMA.pdf
Plesase note that on page 9 there is also the consideration
of the following statement:
“each author had full access to all data”
Response to Comment #3 About Adverse Events from Reviewer#87 JAACAP (2001)
Authors/Linden’s Summary of Comment #3:
“How did the authors’ determine that most of the adverse events in the paroxetine arm were not due to the medication? It is interesting that this arm had the highest rate of psychiatric adverse events, including emotional lability with suicidal ideation and gestures as well as conduct problems and/or hostility. Seven patients in this arm were hospitalized for these difficulties. These numbers are certainly higher than the rate of these problems found in the other two arms. Treatment with SSRIs has been associated with increased irritability as well as the more controversial reports of increased suicidality. It is not stated how many of the serious psychiatric adverse events in the imipramine or placebo arm required hospitalization. Therefore, it is assumed that only those patients in the paroxetine arm were hospitalized. This is a clinically significant finding and needs to be more adequately discussed”
Highlights of Authors/Linden’s Response:
“The causality of adverse events, including serious adverse events, was rated by individual Investigators.”
“We did not intend to deceive or not disclose information. In fact, the manuscript clearly states (page 16 first full paragraph) that 3 of the 5 patients in the imipramine arm experienced serious psychiatric adverse events, 2 of whom were hospitalized. None of the placebo patients were hospitalized. We do not believe that given these small numbers, statements about clinically significant differences are responsible. We therefore wish to retain this portion of the text.”
http://dida.library.ucsf.edu/tid/bpu38h10
Sent to Wagner, Strober, Keller, Ryan, and Linden (2001)
“Both letters comment on the fact that events associated with suicidal ideation/gestures and various aggressive acts were deemed by the treating investigators not to be related to treatment. They question support for such a view. Given that this is medical judgment, I would very much appreciate your help in formulating a response.”
http://dida.library.ucsf.edu/tid/zuu38h10
(No Access to Letter Eventually Sent to JAACAP As Is Behind JAACAP Paywall)
See Comment 6 and 7 from JAMA Reviewer #1 on page 5 regarding issues about the quality of the blind
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/ResponsestoJAMA.pdf
———————————– —————————————
FDA Documents Subsection
Here is a letter from Katz at the FDA in 2002 in relation to GSK’s application for a pediatric indication for Paxil for OCD. Page 2. (2002)
‘8. Please provide your rationale for coding suicide attempts and other forms of self-injurious behavior under the WHOART term “emotional lability”’
Also note that Katz makes the following statement as well, page 1:
“Given the fact that negative trials are frequently seen, even for antidepressant drugs that we know are effective, we agree that it would not be useful to describe these negative trials in labeling.”
http://dida.library.ucsf.edu/tid/esu38h10
One of SKB’s responses to FDA; see pages 3-4 for breakdown of emotional lability events and investigators (2003)
http://dida.library.ucsf.edu/tid/auu38h10
For reference purposes another later document sent to the FDA by GSK, see page 16 (parsing of this data not presented by POGO or Healthy Skepticism, but contains patient identification numbers, as does the document above) (2003)
http://dida.library.ucsf.edu/tid/esu38h10
There are other relevant documents to the FDA correspondence not listed here
—————————— —————————-
Ryan Likely Wrote The First Draft of the Response to the Jeredini Letter (2002)
http://dida.library.ucsf.edu/tid/zru38h10
McCafferty Suggests They Not Address the “Covert” Issues
http://dida.library.ucsf.edu/tid/puu38h10
Strober Endorses the Letter
http://dida.library.ucsf.edu/tid/fuu38h10
Keller and Wagner and Birmaher Endorse the Letter
http://dida.library.ucsf.edu/tid/guu38h10
The Reference To The Emperor Stays In Over McCafferty’s Objection
http://dida.library.ucsf.edu/tid/ouu38h10
Re Ryan and colleagues approaching GSK for further funding, and their characterization of Study 329 (2002)
“As you had suggested in our recent phone conversation, Dr. Birmaher, Dr. Ryan and I are proposing some post hoc analyses of the recently published study of paroxetine in adolescents with major depressive disorders (MDD) (Keller et al., 2001). Dr. Birmaher had presented some interesting findings at last year’s American Psychiatric Association meeting about the effect of comorbid disruptive behavior disorders on treatment response in the trial (Birmaher et al., 2000). We would like to reconsider these findings with a few additional statistical analyses, hoping to disseminate them to a larger clinical and research audience.”
“The recent paper (Keller et al., 2001) reported that paroxetine demonstrated efficacy over placebo in the overall sample, while imipramine did not. Additional analysis about the treatment responses of these comorbid subgroups would be of great interest to clinicians and researchers alike, and give Glaxo Smith-Kline the chance to remind practitioners that this study demonstrated paroxetine’s efficacy in an arguably more representative, comorbid sample of adolescents with MDD”
“Glaxo Smith Kline will certainly have the chance to review any abstracts or papers from these analyses before they are submitted for peer review and publication”
“We are excited about the possibility to shed further light on what has already been a landmark study in our field, and look forward to further discussion with you and others at Glaxo Smith Kline about these proposed analyses.”
http://dida.library.ucsf.edu/tid/vru38h10
Correspondence involving Ryan, Keller, Wagner (Emslie?) responding to GSK’s possible interest in distancing themselves from Study’s 329’s Initial Presentation of Information.(2004)
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/20040204RyantoKellerStrober.pdf
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/040514KellertoCarp.pdf
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/20040413KellertoRyanetal.pdf
Ryan in a Q&A in 2004(?)
“Q: So why has there been such a focus on increased suicide risk among teens taking antidepressants?
A:The question of increased suicide risk is an important and difficult question that needs to be answered.
It is also important to keep in mind that by regulatory precedent, side-effects are lumped into groups such as “emotional lability” but these may not be related to the medication. We know that teens are already at increased risk for suicide. In 2001, it was the third leading cause of death for this age group. In some studies there is actually a correlation between the use of SSRIs and decreased suicide risk.
Q: Do you think the prescription of antidepressants by family MDs and pediatricians are part of the problem?
A:No. I think it is critical that we find treatments that primary doctors can use. They are the 1st line of therapy for uncomplicated depression.
http://psychiatry.ufl.edu/media-center/newsletters/Archive/Ryan/ryan.html
Correction:
Note that in the ‘Keller letter to SKB regarding a naturalistic phase of study 329 (1993)’ comment that after: “Enclosed are some items we discussed regarding the naturalistic phase of the unipolar depression study in adolescents. Your interest and enthusiasm to fund this phase of the study is terrific. You and your colleagues at SmithKline Beecham will be part of the world’s largest and most comprehensive database on adolescent depression.” should come:
Handwritten note in margin: “A little overstated! I agreed to pursue internally”
Annonymous, if you’re the same Anonymous who wrote this at http://1boringoldman.com/index.php/2012/08/22/to-make-distortion-possible/
“I remain unconvinced that the study should be retracted…. To my mind, that emperor has clothes.”
Do you still think the study should not be retracted, even though GSK itself was not satisfied with the results?
Ryan’s intentions, which may even have been altruistic, do not compensate for jiggering the data and recommending a drug with serious risks for children to pediatricians.
Sent to Wagner, Strober, Keller, Ryan, and Linden (2001)
“Both letters comment on the fact that events associated with suicidal ideation/gestures and various aggressive acts were deemed by the treating investigators not to be related to treatment. They question support for such a view. Given that this is medical judgment, I would very much appreciate your help in formulating a response.”
http://dida.library.ucsf.edu/tid/zuu38h10
(No Access to Letter Eventually Sent to JAACAP As Is Behind JAACAP Paywall)
See Comment 6 and 7 from JAMA Reviewer #1 on page 5 regarding issues about the quality of the blind
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/ResponsestoJAMA.pdf
FDA Documents Subsection
Here is a letter from Katz at the FDA in 2002 in relation to GSK’s application for a pediatric indication for Paxil for OCD. Page 2. (2002)
‘8. Please provide your rationale for coding suicide attempts and other forms of self-injurious behavior under the WHOART term “emotional lability”’
Also note that Katz makes the following statement as well, page 1:
“Given the fact that negative trials are frequently seen, even for antidepressant drugs that we know are effective, we agree that it would not be useful to describe these negative trials in labeling.”
http://dida.library.ucsf.edu/tid/esu38h10
One of SKB’s responses to FDA; see pages 3-4 for breakdown of emotional lability events and investigators (2003)
http://dida.library.ucsf.edu/tid/auu38h10
For reference purposes another later document sent to the FDA by GSK, see page 16 (parsing of this data not presented by POGO or Healthy Skepticism, but contains patient identification numbers, as does the document above) (2003)
http://dida.library.ucsf.edu/tid/esu38h10
There are other relevant documents to the FDA correspondence not listed here
Ryan Likely Wrote The First Draft of the Response to the Jeredini Letter (2002)
http://dida.library.ucsf.edu/tid/zru38h10
McCafferty Suggests They Not Address the “Covert” Issues
http://dida.library.ucsf.edu/tid/puu38h10
Strober Endorses the Letter
http://dida.library.ucsf.edu/tid/fuu38h10
Keller and Wagner and Birmaher Endorse the Letter
http://dida.library.ucsf.edu/tid/guu38h10
The Reference To The Emperor Stays In Over McCafferty’s Objection
http://dida.library.ucsf.edu/tid/ouu38h10
Sent to Wagner, Strober, Keller, Ryan and Linden (2001)
“Both letters comment on the fact that events associated with suicidal ideation/gestures and various aggressive acts were deemed by the treating investigators not to be related to treatment. They question support for such a view. Given that this is medical judgment, I would very much appreciate your help in formulating a response.”
http://dida.library.ucsf.edu/tid/zuu38h10
Seee comment 6 and 7 from JAMA reviewer #1 on page 5 regarding issues about the quality of the blind
http://www.healthyskepticism.org/files/docs/gsk/paroxetine/study329/ResponsestoJAMA.pdf
FDA Documents Subsection
Here is a letter from Katz at the FDA in 2002 in relation to GSK’s application for Pediatric OCD indication for Paxil. Page 2. (2002)
‘8. Please provide your rationale for coding suicide attempts and other forms of self-injurious behavior under the WHOART term “emotional lability”’
Also note that Katz makes the following statement as well, page 1:
“Given the fact that negative trials are frequently seen, even for antidepressant drugs that we know are effective, we agree that it would not be useful to describe these negative trials in labeling.”
http://dida.library.ucsf.edu/tid/esu38h10
One of SKB’s responses to FDA; see pages 3-4 for some breakdown of adverse events and investigators (2003)
http://dida.library.ucsf.edu/tid/auu38h10
For reference purposes another later document sent to the FDA by GSK, see page 16 (parsing of this data not presented by POGO or Healthy Skepticism, but contains patient identification numbers, as does the document above) (2003)
http://dida.library.ucsf.edu/tid/esu38h10
There are other relevant documents to the FDA correspondence on the site not listed here.
Ryan Appears to Have Written The First Draft of the Response to the Jeredini Letter (2002)
http://dida.library.ucsf.edu/tid/zru38h10
McCafferty Suggests They Not Address the “Covert” Issues
http://dida.library.ucsf.edu/tid/puu38h10
Strober Endorses the Letter
http://dida.library.ucsf.edu/tid/fuu38h10
Keller and Wagner and Birmaher Endorse the Letter
http://dida.library.ucsf.edu/tid/guu38h10
The Reference To The Emperor Stays In Apparently Over McCafferty’s Objection
http://dida.library.ucsf.edu/tid/ouu38h10
http://dida.library.ucsf.edu/tid/zru38h10
Ryan appears to have written the first draft of the response to the Jureidinin letter (2002)
McCafferty Suggests They not Address the “Covert” Issues
http://dida.library.ucsf.edu/tid/puu38h10
http://dida.library.ucsf.edu/tid/fuu38h10
Strober Endorses the Letter
Keller and Wagner and Birmaher Endorse the Letter http://dida.library.ucsf.edu/tid/guu38h10
The Reference To The Emperor Stays in Apparently Over McCafferty’s Objection
http://dida.library.ucsf.edu/tid/ouu38h10
Correspondence between Ryan, Keller, Wagner (Emslie?) responding to GSK’s possible interest in distancing themselves from Study’s 329’s Initial Presentation of Information.(2004)
3pdfs at the Healthy Skepticism 329 Site:
20040204RyantoKellerStrober
040514KellertoCarp
20040413KellertoRyanetal
20040413KellertoRyanetal
FDA Documents Subsection Part 1
Here is a letter from Katz at the FDA in 2002 in relation to GSK’s application for a pediatric indication for Paxil for OCD. Page 2. (2002)
‘8. Please provide your rationale for coding suicide attempts and other forms of self-injurious behavior under the WHOART term “emotional lability”’
Also note that Katz makes the following statement as well, page 1:
“Given the fact that negative trials are frequently seen, even for antidepressant drugs that we know are effective, we agree that it would not be useful to describe these negative trials in labeling.”
http://dida.library.ucsf.edu/tid/esu38h10
FDA Documents Subsection Part 2
One of SKB’s responses to FDA; see pages 3-4 for breakdown of emotional lability events and investigators (2003)
http://dida.library.ucsf.edu/tid/auu38h10
For reference purposes another later document sent to the FDA by GSK, see page 16 (parsing of this data not presented by POGO or Healthy Skepticism, but contains patient identification numbers, as does the document above) (2003)
http://dida.library.ucsf.edu/tid/esu38h10
There are other relevant documents of the FDA correspondence on the site not listed in these subsections.
FDA Documents Subsection Part 2
http://dida.library.ucsf.edu/tid/auu38h10
One of SKB’s responses to FDA; see pages 3-4 for breakdown of emotional lability events and investigators (2003)
http://dida.library.ucsf.edu/tid/esu38h10
For reference purposes another later document sent to the FDA by GSK, see page 16 (parsing of this data not presented by POGO or Healthy Skepticism, but contains patient identification numbers, as does the document above) (2003)
There are other relevant documents of the FDA correspondence on the site not listed in these subsections.
One of SKB’s responses (there are others at the POGO site not parsed) to FDA (2003). Look for example at pages 3-4.
http://dida.library.ucsf.edu/tid/auu38h10
There are also breakdowns of the details of some of the serious adverse psychiatric events in the appendices of some of the over 100page long internal SKB reports that got genereated through study 329.
Who are the Guardians of the Profession?
“You have not bee appointed as the guardian of the journal, or of the profession of child psychiatry. Many highly expert child and adolescent psychiatrists were participants in that study, and others that were similar, and others equally expert contributed to the review process. In addition, the prescription of SSRIs for youth, not only in this country but around the world, far predated any research data on their effects with youth, so this article could hardly be praised or blamed for that trend.” – Mina Dulcan
“Science is not run by democracy” – Rachel Klein
http://dida.library.ucsf.edu/tid/hvu38h10
SKB Beebe to Ryan Describing the Meaning of “emotional lability” Label (1998)
(SKB McCafferty cced on this as well)
“Plese note that in the Adverse Experiences Table (14,2.1), under the heading “Nervous System,” the categories Emotional Lability and Euphoria refer to suicidality and mania/hypermania respectively.”
http://dida.library.ucsf.edu/tid/xou38h10
Table of adverse events and assessed relatedness.
http://dida.library.ucsf.edu/tid/aru38h10
It does not appear that clear criteria for how relatedness was determined (and was assured integrity of the blind) and how many were withdrawn from the study secondary to these issues has ever been clearly detailed.
The 1998 Wagner Poster (she appears to have been the primary presenter on safety) is at the Healthy Skepticism site.
The 2000 Wagner SKB produced Poster is part of the POGO documents (Table 5 is on Page 10)
http://dida.library.ucsf.edu/tid/dvu38h10
Geller to Ryan (1999) re how Imipramine data was being presented
“i suspect my comment has already received attention, but it is one that I fully believe must be addressed in the abstract because not to do so seems deceptive, notice that this e-mail goes only to you, although we were asked to send to you, Mike and Marty plus Sally. the abstract does not include that IMJ HAD to reach at least 200 mg/day. the sentence on IMI dosage under the paragraph title intervention, must be amended to state that ” was gradually titrated upwards FROM A MINIMUM OF 2000 MG/DAY based on …” will leave this in your capable hands to get inserted, this is a key point because of tne observation that some of the IMI subjects had responded at lower than 200 mg/day and that the IMI toxicity was higher with increasing dose clinically.”
http://dida.library.ucsf.edu/tid/ppu38h10
See related comment from reviewer #1 on page 5 of the 1999-2000 JAMA reviewer documents noted earlier.
Reviewer #1 on page 5 can be seen at ResponsestoJAMA.pdf at the Healthy Skepticism 329 website.
SKB’s McCafferty Expressing Concern to Ryan, Keller, Strober, Linden regarding reporting of side effects (March 1999)
“A reviewer may question the adverse events profile for paroxetine. Although most events reported were similar in nature and frequency to that reported in the adult, there were higher number of paroxetine patients who reported “emotional liability” and “hostility”. The term “emotional liability” was catch all term for “suicidal ideation and gestures”. The hostility term captures behavioral problems, most related to parental and school confrontations. Although the investigators did not feel this was a problems, it may raise a flag if not described.”
http://dida.library.ucsf.edu/tid/uuu38h10
http://dida.library.ucsf.edu/tid/rvu38h10
The second link is Ryan forwarding the email to Keller. This email from McCafferty dated in March 1999. Klein’s correspondence with McCafferty on reporting of adverse events is April 1999.
SKB’s McCaffery Expressing Concern to Ryan, Keller, Strober, Linden
(Ryan forwarded the information to Keller) Regarding the Reporting of Side Effects (March 1999)
SKB’s McCafferty Expressing Concern to Ryan, Keller, Strober, Linden
Regarding the Reporting of Side Effects (March 1999)
“A reviewer may question the adverse events profile for paroxetine. Although most events reported were similar in nature and frequency to that reported in the adult, there were higher number of paroxetine patients who reported “emotional liability” and “hostility”. The term “emotional liability” was catch all term for “suicidal ideation and gestures”. The hostility term captures behavioral problems, most related to parental and school confrontations. Although the investigators did not feel this was a problems, it may raise a flag if not described.”
http://dida.library.ucsf.edu/tid/uuu38h10
“A reviewer may question the adverse events profile for paroxetine. Although most events reported were similar in nature and frequency to that reported in the adult, there were higher number of paroxetine patients who reported “emotional liability” and “hostility”. The term “emotional liability” was catch all term for “suicidal ideation and gestures”. The hostility term captures behavioral problems, most related to parental and school confrontations. Although the investigators did not feel this was a problems, it may raise a flag if not described.”
McCafferty Expressing Concern to Linden Regarding the Reporting of Side Effects (July 19th 1999)
“I reviewed the latest version of the manuscript and have some comments. Two are minor but one is of some concern.”
“Major.
Safety. It seems incongruous that we state that paroxetine is safe yet report so many SAEs. I know the investigators have not raised an issue, but I fear that the editors will. I am still not sure how to describe these events. I will again review all the SAEs to make myself feel comfortable about what we report in print.”
http://dida.library.ucsf.edu/tid/tuu38h10
Further Letter to Linden 3 Days Later (July 21st 1999)
“Because the number of patients with serious AEs reported for the paroxetine group (11/93, 12%) is high vis a vis the placebo group (2/87, 2%), additional text may be necessary for prospective. I would provide some definition of an SAE, and also state that the serious events, with few exceptions, were psychiatric in nature(as opposed to physical). We should also describe what events led to hospitalization (n.b. 7 paxil patients not 6). Some suggested text below.”
http://dida.library.ucsf.edu/tid/ssu38h10
McCafferty may have continued to have had concerns
(Linden and Keller’s Response) (2000)
“Today, Jim McCafferty asked us to hold this manuscript until the SB legal department can review it one last time.”
http://dida.library.ucsf.edu/tid/bvu38h10
Should acknowledge that Draft 1 went to SKB and only Draft 2 went to the authors.
http://dida.library.ucsf.edu/pdf/uru38h10
Should also include this, though not directly related, as it is an issue I know 1BOM has focused on in the past:
http://dida.library.ucsf.edu/pdf/kmu38h10