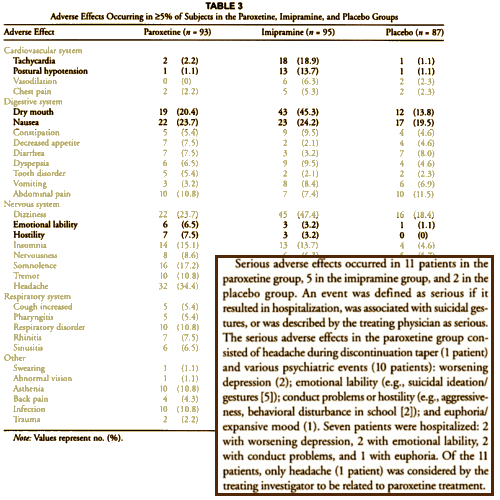
This is how the Adverse Events were presented in the article: the well known CV Effects for Imipramine; the annoying dry mouth from Imipramine’s anticholinergic effects; and a little something in the Nervous System section under Paroxetine. In the text, things look a bit more ominous though it’s a little hard to parse out what they’re saying the way it’s written. emotional lability apparently is a synonym for suicidality. But after that, there are 11 patients with serious adverse effects, 21 instances of serious adverse effects, 5 suicidal patients, 7 hospitalized, but only 1 [a headache] related to the medication? Pretty damn confusing. It seems to have confused others too. So by the time it got to the DOJ suit this year [11 years after the article was published], much more had been revealed by various queries. This is from the DOJ Complaint in the recent suit [the $3B settlement suit!] to which GSK agreed without reservation:
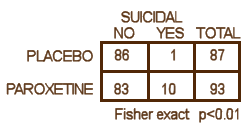 We have little choice but to accept these DOJ figures. Apparently, GSK did, or they wouldn’t have paid $3B to settle the suit. But what if the DOJ figures were off as well? How many suicide related events would’ve been considered significant. The graph on the left shows the number of events and their probability of being significant. The answer is 8 or more to achieve significance, and I accept there were at least that many. So in their list of adverse events in the table,
We have little choice but to accept these DOJ figures. Apparently, GSK did, or they wouldn’t have paid $3B to settle the suit. But what if the DOJ figures were off as well? How many suicide related events would’ve been considered significant. The graph on the left shows the number of events and their probability of being significant. The answer is 8 or more to achieve significance, and I accept there were at least that many. So in their list of adverse events in the table, 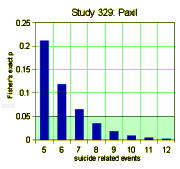 suicide related events are listed as emotional lability and under-reported by as much as 50%. As in the case of efficacy, this looks for all the world like a specific attempt to mislead the readers rather than some mistake or piece of sloppiness. At this juncture, it would be easy to go off on a detour for another round of righteous indignation, and it would sure be justified because this article actively
suicide related events are listed as emotional lability and under-reported by as much as 50%. As in the case of efficacy, this looks for all the world like a specific attempt to mislead the readers rather than some mistake or piece of sloppiness. At this juncture, it would be easy to go off on a detour for another round of righteous indignation, and it would sure be justified because this article actively minimized hid the significant finding that over 10% of the subjects taking Paroxetine experienced a suicide related event [and at least 7.5% experienced "hostility" – probably more].
 Likewise, there’s the issue of whodunit which I find intriguing [and important] mentioned in fascinating anonymous comments recently [here, here, here, etc]. I hope that information continues to come [so long as it passes the test of "evidence based"]. Was it Ryan and Keller in the Parlor? Dulcan in the Conservatory? Sally Laden in the Garden? GSK in the Pantry? Corporate greed? Therapeutic Zeal? Ideology? These things certainly matter and I’ll get to those questions. But right now I’m focused on something much simpler. Eleven years after it’s publication, we’re engaged in an almost unending debate about whether the new antidepressants are safe to give to adolescents – to wit, the recent articles by Gibbons et al spoken about so often here. As a clinician, I know they’re not altogether safe, at least not without warning and careful monitoring. I’ve only seen adolescents as a retired person [since 2007-ish], but I’ve personally seen enough cases of "hostility" and "suicide related events" on SSSRIs to know that it happens and that it’s idiosyncratic, not part of the illness that brought the patient for care. So here’s Study 329 that would’ve helped us with that debate a long time ago, but didn’t. Right now, I want to look at the belatedly posted raw data from the study to see if it would’ve been enough to help us with this important clinical question had it been available in 2001. And if not, what’s still missing?
Likewise, there’s the issue of whodunit which I find intriguing [and important] mentioned in fascinating anonymous comments recently [here, here, here, etc]. I hope that information continues to come [so long as it passes the test of "evidence based"]. Was it Ryan and Keller in the Parlor? Dulcan in the Conservatory? Sally Laden in the Garden? GSK in the Pantry? Corporate greed? Therapeutic Zeal? Ideology? These things certainly matter and I’ll get to those questions. But right now I’m focused on something much simpler. Eleven years after it’s publication, we’re engaged in an almost unending debate about whether the new antidepressants are safe to give to adolescents – to wit, the recent articles by Gibbons et al spoken about so often here. As a clinician, I know they’re not altogether safe, at least not without warning and careful monitoring. I’ve only seen adolescents as a retired person [since 2007-ish], but I’ve personally seen enough cases of "hostility" and "suicide related events" on SSSRIs to know that it happens and that it’s idiosyncratic, not part of the illness that brought the patient for care. So here’s Study 329 that would’ve helped us with that debate a long time ago, but didn’t. Right now, I want to look at the belatedly posted raw data from the study to see if it would’ve been enough to help us with this important clinical question had it been available in 2001. And if not, what’s still missing?
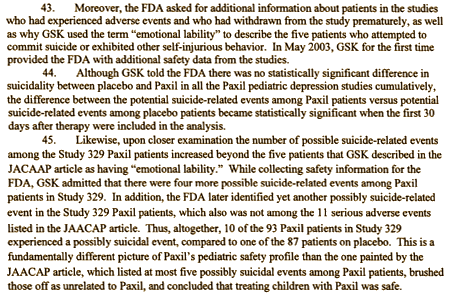
I would suggest that the information contained within the POGO/UCSF documents are relevant to the question of the safety data being easily accessible to those who could benefit from it. Ryan, Keller, and Wagner were physicians who had arguably the greatest responsibility and ability to make sure this was available. And, their impact on this prior to, during, and after submission of the paper (even once GSK itself appeared interested in this being more straightforwardly reported in 2004) can be assessed from the links I posted.
The author list on the 2001 paper is long. There are many individuals at SKB/GSK with whom we are not familiar. Dulcan makes reference to many “highly expert” individuals. SKB/GSK would have had much greater difficulty trumpeting the high safety of Paxil to primary care providers without this 2001 paper authored by a whos who in child psychiatry and published in their flagship journal.
That is not an issue of simply academic concern.
Dulcan could have forced them to either forthrightly report the adverse reaction data as so eliquently laid out by JAMA reviewer #2 (she did not he access to that) and JAACAP reviewer #87 (she did have access to that) or be forced to not have it published in JAACAP.
She did not.
Those reviewers in 1999 did not need additional data or the lens of over a decade of debate to recognize that this data needed to be represented more forthrightly. That is central to the availability that you seek and could have been achieved by editors at the time with the tools available to them.
The editor at JAMA managed to do it.
There is a certain irony in her statement to Healthy Skepticism that they had not been appointed guardians of the journal, or child psychiatry.
For going on 50 years JAACAP editors appear to have served for 10 years apiece. AACAP has had the same executive director for going on 40 years.
As Dr. Klein said “Science is not run as a democracy.”
I believe that the availability of the POGO documents allow us to examine the impact of that fact, and the behavior of the key guardians.
Because the old guardians are the new guardians.
Many “highly expert” individuals tried to raise these issues over a decade ago (as detailed in the links I posted). That had limited to no impact on Drs. Ryan and Dulcan and their roles within child psychiatry, including JAACAP/AACAP.
Why would now be any different?
Annonymous,
Thanks for the links. As I said in this post, I’ll get to their misbehavior soon myself and your information is a big help. Your perspective on the roles [and failures] of the psychiatrists involved is noted and appreciated. I knew of the others, but hadn’t realized Dr. Ryan’s major role in this story. When I get there, I hope you’ll add your piece too – either as a guest contributer or in the comments.
“Why would now be any different?”
So far, it wouldn’t.
Unfortunately, there’s a contemporary set of articles on the table virtually on the same topic as Study 329 – the Gibbons et al papers on efficacy and safety of SSRIs in kids [Suicidal Thoughts and Behavior With Antidepressant Treatment, Benefits From Antidepressants]. They’ve been widely publicized and the Journal Editor has refused to accept letters pointing out their obvious foibles for full publication [Archives of General Psychiatry]. Like Study 329, the Raw Data is not available for independent analysis, so we are left making indirect criticisms that could be made much more forcefully if we had access to the data. The reason I’m looking at the [finally] available raw data of 329 is to make the point that with that access, one can identify deceitful science and mount publishable counterarguments.
You are correct that Drs.Keller, Ryan, Wagner, Dulcan, and others abused their power and used secrecy with 329. No argument. My point is that with full data transparency, such things would be much harder to pull off, much easier to vet and expose. So hopefully, the cry “remember Study 329“ will carry some weight in the future. If my point turns out to be naive, I’ll be glad to publish your contrary argument.
Thank you for taking the time to respond. I would hope to contribute to your efforts rather than look to attempt a contrary argument.
Here is my attempt:
Focus on the serious psychiatric event data for paroxetine rather than on the issue of efficacy or lack thereof. Follow the story of that raw data, and then after that on what Drs. Keller, Ryan, Wagner possible impact on what GSK then did or did not do to disseminate information about that data.
Begin to explore the following documents:
Study 329 had 2 components: an 8 week acute phase assessing treatment effects and a 6 month continuation phase to assess long-term safety of therapy (along with some other objectives) in responders.
http://dida.library.ucsf.edu/tid/upu38h10
So the safety data comes from the acute phase and the continuation phase.
This is the Study 329 Reporting and Analysis Plan
http://dida.library.ucsf.edu/tid/znu38h10
This lets you know which tables you care about most:
Table 14.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Acute Phase)
Table 14.8 Listing of Serious Adverse Experiences (Acute Phase)
Table 16.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Continuation Phase)
Table 16.8 Listing of Serious Adverse Experiences (Continuation Phase)
There is also a slightly revised RAP but the table listings stay the same
http://dida.library.ucsf.edu/tid/pvu38h10
Here is Table 48 with the Acute Phase Individuals
http://dida.library.ucsf.edu/tid/aru38h10
(derived from 14.8 which I suspect you can also find if you dig around as well as the appendixes references at the bottom of the table)
See also page 112 of http://dida.library.ucsf.edu/tid/gnu38h10
And Here is Table 16.8 with the Continuation Phase Adverse Events
page 1
http://dida.library.ucsf.edu/tid/eru38h10
page 2
http://dida.library.ucsf.edu/tid/fru38h10
See also
http://dida.library.ucsf.edu/tid/dru38h10
and
http://dida.library.ucsf.edu/tid/cru38h10
By April 1998 Ryan appears to have had both the acute phase and continuation data
http://dida.library.ucsf.edu/tid/xou38h10
The 1998 Wagner Poster that lists the withdrawal % for the paxil group
WagnerPoster.pdf at the Healthy Skepticism Site
This was an SKB produced poster (I apologize, I mistakenly labeled this in an earlier comment as being from 2000)
http://dida.library.ucsf.edu/tid/dvu38h10
Look at Table 5. There are 9 adolescents (with identifiers) in the Paxil arm listed as hospitalized or overdose in the acute phase alone. 2 similar events in the imipramine arm and 2 similar events in the placebo arm.
Klein’s Query to McCafferty about possible discrepancies
http://dida.library.ucsf.edu/tid/lpu38h10
This SKB response to the FDA, see pages 3-4
http://dida.library.ucsf.edu/tid/auu38h10
Then there is the correspondence involving Keller, Ryan, Strober, Wagner, and Emslie regarding GSK’s apparent plan to publish a review paper regarding the safety data
Healthy Skepticism Study 329 site
20040204RyantoKellerStrober.pdf
040514KellertoCarp.pdf
20040413KellertoRyanetal.pdf
Keller states in the June 2004 to Ryan, Strober, Emslie, and Wagner:
“we are not without leverage if we are dissatisfied and want to play a little hardball of our own.”
“if we want to fight that battle we will have to go to senior management. I am willing to do that if we agree that after the changes are made to this draft we are still hung out to dry.”
“so that it is 100% clear in this paper that there is no way to read it and think that 329 is being criticized and that it was not written with complete integrity and accuracy given the data we had and should have had as investigators, on the part of investigators and our collegues from SK who worked on it.”
“otherwise we would look foolish, naive, incompetent, or “biased” (the most likely accusation that will be made) to present things in a way that was favorable to SK, disregarding our responsibility to the proper scientific method, to the public, children and their families.”
After Keller discussed going to more senior management in October 1999 about SKB McCafferty’s holding up the 329 JAACAP manuscript, in November 1999 the manuscript was submitted.
In April 2004 GSK’s Carpenter is stating “there is a corporate need/obligation to communicate the key safety and efficacy data from all of these studies to the medical community in a manner which is consistent with the newly revised labeling ASAP. Consequently we have drafted a “review” manuscript which we hope to submit for publication very soon (attached below for your review). This paper presents the key efficacy and safety data for each of the studies individuallyl and it also presents the results of analyses of the pooled dataset (all three studies combined).”
In June 2004 Keller then discusses possibly going to senior management if “dissatisfied.” This apparently out of concern that they could be viewed as “disregarding our responsibility to the proper scientific method, to the public, children and their families.”
My best guess is that the review paper turned out to be this one that came out in 2006: 16553530 in pubmed. I’m not sure how one would get access, but if you can it seems like it would be worth looking at the data they would present.
The available documents don’t really allow us to know what that review would have looked like if GSK had not received Keller et al’s feedback.
I am in total agreement that there needs to be greater data transparency. I am suggesting that after you parse the data more fully you consider taking a page from Allen Frances’ playbook. He realized that addressing himself to the APA was a losing proposition so he went to a broader audience to present a consistent drumbeat regarding poor science, secrecy, and hubris. Most importantly, he tried to address the DSM V’s target audience, its customers if you will. There are many more non-psychiatric users of the DSM that psychiatry end-users.
There are let’s say about 8000 practicing child psychiatrists in the US. There are about 60,000 pediatricians and about 100,000 family practitioners (I’m not clear if these numbers even include other clinicians with prescribing privileges such as NPs). At the very least we are talking a 20 fold difference.
That is why SKB went after JAMA first. That is why the APA is paying attention to Frances (to the extent they are at all).
Parse the raw data. I would strongly recommend that you focus on the safety data. Because overselling efficacy isn’t a shock to many people. JAMA reviewer #2 highlighted, however, the obligation to look out for the primary care provider and give them the data they need to make informed decisions.
It does not appear that Drs. Ryan,Wager, Dulcan, and of course Keller, believed that those physicians could be trusted to make informed decisions if presented the raw adverse reaction data forthrightly. Whatever in the long run turns out to be the correct way of conceptualizing the possible side effects of paroxetine. At the very least you’d know what to look out for and counsel around, since it’s very hard to look at that data and not think that there is at the very least some greater propensity for psychiatric side effects. Don’t tell me about the possible side effects I should look for because I might be too concerned to prescribe? Because that wouldn’t be “responsible”? I think (hope?) that that would resonate with primary care physicians. And if primary care physicians start being wary of JAACAP/AACAP based practice recommendations, that will be something that would likely trigger an engagement in a much more genuine discussion about the issues we are discussing in this small back room of cyberspace. Truly, no offense meant to 1BOM.
It might also be hard for them to read Keller’s comments and imagine that they were discussing using “hardball” with GSK to influence GSK to act as much as possible in the best interest of children and their families and the physicians relying on the resultant information to try to manage their treatment.
Put everything together and see if there is any forum in the journal Pediatrics that would let you present it? One can always dream.
Maybe those physicians would begin to wonder if the current system of data ownership and publication, particularly as practiced within child psychiatry is best serving their needs. And how much stock they wish to place in the next set of recommendations endorsed by the handful of primary individuals involved in Study 329.
I hope you are successful in bringing attention to the lack of access to raw data (particularly safety data).
Best of luck to you.
Here is a tip: when you access the UCSF/POGO archive, and this is the only way you can start to truly explore the raw data on the adverse events, keep in mind that you can search the archive to find pdfs (and then within the pdfs themselves) using the unique patient identification numbers. For example 329.005.00011 or can also show up sometimes as 0011.
Thank you for having taken the time to respond.
I would hope to contribute to your efforts rather than look to attempt a contrary argument.
Here is my attempt:
Focus on the serious psychiatric event data for paroxetine rather than on the issue of efficacy or lack thereof. Follow the story of that raw data, and then after that on what Drs. Keller, Ryan, Wagner possible impact on what GSK then did or did not do to disseminate information about that data.
Begin to explore the following documents:
Study 329 had 2 components: an 8 week acute phase assessing treatment effects and a 6 month continuation phase to assess long-term safety of therapy (along with some other objectives) in responders.
http://dida.library.ucsf.edu/tid/upu38h10
So the safety data comes from the acute phase and the continuation phase.
This is the Study 329 Reporting and Analysis Plan
http://dida.library.ucsf.edu/tid/znu38h10
This lets you know which tables you care about most:
Table 14.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Acute Phase)
Table 14.8 Listing of Serious Adverse Experiences (Acute Phase)
Table 16.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Continuation Phase)
Table 16.8 Listing of Serious Adverse Experiences (Continuation Phase)
There is also a slightly revised RAP but the table listings stay the same
http://dida.library.ucsf.edu/tid/pvu38h10
Here is Table 48 with the Acute Phase Individuals
http://dida.library.ucsf.edu/tid/aru38h10
(derived from 14.8 which I suspect you can also find if you dig around as well as the appendixes references at the bottom of the table)
See also page 112 of http://dida.library.ucsf.edu/tid/gnu38h10
And Here is Table 16.8 with the Continuation Phase Adverse Events
page 1
http://dida.library.ucsf.edu/tid/eru38h10
page 2
http://dida.library.ucsf.edu/tid/fru38h10
See also
http://dida.library.ucsf.edu/tid/dru38h10
and
http://dida.library.ucsf.edu/tid/cru38h10
By April 1998 Ryan appears to have had both the acute phase and continuation data
http://dida.library.ucsf.edu/tid/xou38h10
The 1998 Wagner Poster that lists the withdrawal % for the paxil group
WagnerPoster.pdf at the Healthy Skepticism Site
This was an SKB produced poster (I apologize, I mistakenly labeled this in an earlier comment as being from 2000)
http://dida.library.ucsf.edu/tid/dvu38h10
Look at Table 5. There are 9 adolescents (with identifiers) in the Paxil arm listed as hospitalized or overdose in the acute phase alone. 2 similar events in the imipramine arm and 2 similar events in the placebo arm.
Klein’s Query to McCafferty about possible discrepancies
http://dida.library.ucsf.edu/tid/lpu38h10
This SKB response to the FDA, see pages 3-4
http://dida.library.ucsf.edu/tid/auu38h10
Then there is the correspondence involving Keller, Ryan, Strober, Wagner, and Emslie regarding GSK’s apparent plan to publish a review paper regarding the safety data
Healthy Skepticism Study 329 site
20040204RyantoKellerStrober.pdf
040514KellertoCarp.pdf
20040413KellertoRyanetal.pdf
Keller states in the June 2004 to Ryan, Strober, Emslie, and Wagner:
“we are not without leverage if we are dissatisfied and want to play a little hardball of our own.”
“if we want to fight that battle we will have to go to senior management. I am willing to do that if we agree that after the changes are made to this draft we are still hung out to dry.”
“so that it is 100% clear in this paper that there is no way to read it and think that 329 is being criticized and that it was not written with complete integrity and accuracy given the data we had and should have had as investigators, on the part of investigators and our collegues from SK who worked on it.”
“otherwise we would look foolish, naive, incompetent, or “biased” (the most likely accusation that will be made) to present things in a way that was favorable to SK, disregarding our responsibility to the proper scientific method, to the public, children and their families.”
After Keller discussed going to more senior management in October 1999 about SKB McCafferty’s holding up the 329 JAACAP manuscript, in November 1999 the manuscript was submitted.
In April 2004 GSK’s Carpenter is stating “there is a corporate need/obligation to communicate the key safety and efficacy data from all of these studies to the medical community in a manner which is consistent with the newly revised labeling ASAP. Consequently we have drafted a “review” manuscript which we hope to submit for publication very soon (attached below for your review). This paper presents the key efficacy and safety data for each of the studies individuallyl and it also presents the results of analyses of the pooled dataset (all three studies combined).”
In June 2004 Keller then discusses possibly going to senior management if “dissatisfied.” This apparently out of concern that they could be viewed as “disregarding our responsibility to the proper scientific method, to the public, children and their families.”
My best guess is that the review paper turned out to be this one that came out in 2006: 16553530 in pubmed. I’m not sure how one would get access, but if you can it seems like it would be worth looking at the data they would present.
The available documents don’t really allow us to know what that review would have looked like if GSK had not received Keller et al’s feedback.
I am in total agreement that there needs to be greater data transparency. I am suggesting that after you parse the data more fully you consider taking a page from Allen Frances’ playbook. He realized that addressing himself to the APA was a losing proposition so he went to a broader audience to present a consistent drumbeat regarding poor science, secrecy, and hubris. Most importantly, he tried to address the DSM V’s target audience, its customers if you will. There are many more non-psychiatric users of the DSM that psychiatry end-users.
There are let’s say about 8000 practicing child psychiatrists in the US. There are about 60,000 pediatricians and about 100,000 family practitioners (I’m not clear if these numbers even include other clinicians with prescribing privileges such as NPs). At the very least we are talking a 20 fold difference.
That is why SKB went after JAMA first. That is why the APA is paying attention to Frances (to the extent they are at all).
Parse the raw data. I would strongly recommend that you focus on the safety data. Because overselling efficacy isn’t a shock to many people. JAMA reviewer #2 highlighted, however, the obligation to look out for the primary care provider and give them the data they need to make informed decisions.
It does not appear that Drs. Ryan,Wager, Dulcan, and of course Keller, believed that those physicians could be trusted to make informed decisions if presented the raw adverse reaction data forthrightly. Whatever in the long run turns out to be the correct way of conceptualizing the possible side effects of paroxetine. At the very least you’d know what to look out for and counsel around, since it’s very hard to look at that data and not think that there is at the very least some greater propensity for psychiatric side effects. Don’t tell me about the possible side effects I should look for because I might be too concerned to prescribe? Because that wouldn’t be “responsible”? I think (hope?) that that would resonate with primary care physicians. And if primary care physicians start being wary of JAACAP/AACAP based practice recommendations, that will be something that would likely trigger an engagement in a much more genuine discussion about the issues we are discussing in this small back room of cyberspace. Truly, no offense meant to 1BOM.
It might also be hard for them to read Keller’s comments and imagine that they were discussing using “hardball” with GSK to influence GSK to act as much as possible in the best interest of children and their families and the physicians relying on the resultant information to try to manage their treatment.
Put everything together and see if there is any forum in the journal Pediatrics that would let you present it? I realize I am being naive.
Maybe those physicians would begin to wonder if the current system of data ownership and publication, particularly as practiced within child psychiatry is best serving their needs. And how much stock they wish to place in the next set of recommendations endorsed by the handful of primary individuals involved in Study 329.
I hope you are successful in bringing attention to the lack of access to raw data (particularly safety data).
Best of luck to you.
Here is a tip: when you access the UCSF/POGO archive, and this is the only way you can start to truly explore the raw data on the adverse events, keep in mind that you can search the archive to find pdfs (and then within the pdfs themselves) using the unique patient identification numbers. For example 329.005.00011 or can also show up sometimes as 0011.
Thank you for taking the time to respond. I would hope to contribute to your efforts rather than look to attempt a contrary argument.
Here is my attempt:
Focus on the serious psychiatric event data for paroxetine rather than on the issue of efficacy or lack thereof. Follow the story of that raw data, and then after that on what Drs. Keller, Ryan, Wagner possible impact on what GSK then did or did not do to disseminate information about that data.
Begin to Explore The Following Documents
Study 329 had 2 components: an 8 week acute phase assessing treatment effects and a 6 month continuation phase to assess long-term safety of therapy (along with some other objectives) in responders.
http://dida.library.ucsf.edu/tid/upu38h10
So the safety data comes from the acute phase and the continuation phase.
This is the Study 329 Reporting and Analysis Plan
http://dida.library.ucsf.edu/tid/znu38h10
This lets you know which tables you care about most:
Table 14.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Acute Phase)
Table 14.8 Listing of Serious Adverse Experiences (Acute Phase)
Table 16.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Continuation Phase)
Table 16.8 Listing of Serious Adverse Experiences (Continuation Phase)
There is also a slightly revised RAP but the table listings stay the same
http://dida.library.ucsf.edu/tid/pvu38h10
This is the Study 329 Reporting and Analysis Plan
http://dida.library.ucsf.edu/tid/znu38h10
This lets you know which tables you care about most.
Table 14.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Acute Phase)
Table 14.8 Listing of Serious Adverse Experiences (Acute Phase)
Table 16.2 Summary of Adverse Experiences by ADECS Body System and Preferred Term (Continuation Phase)
Table 16.8 Listing of Serious Adverse Experiences (Continuation Phase)
There is also a slightly revised RAP but the table listings stay the same
http://dida.library.ucsf.edu/tid/pvu38h10
Here is Table 48 with the Acute Phase Individuals
http://dida.library.ucsf.edu/tid/aru38h10
(derived from 14.8 which I suspect you can also find if you dig around as well as the appendixes references at the bottom of the table)
See also page 112 of of http://dida.library.ucsf.edu/tid/gnu38h10
Here is Table 48 with the Acute Phase Individuals
http://dida.library.ucsf.edu/tid/aru38h10
(derived from 14.8 which I suspect you can also find if you dig around as well as the appendixes references at the bottom of the table)
See also page 112 of
http://dida.library.ucsf.edu/tid/gnu38h10
And Here is Table 16.8 with the Continuation Phase Adverse Events
page 1 http://dida.library.ucsf.edu/tid/eru38h10
page 2 http://dida.library.ucsf.edu/tid/fru38h10
And Here is Table 16.8 with the Continuation Phase Adverse Events
page 1 http://dida.library.ucsf.edu/tid/eru38h10
page 2 http://dida.library.ucsf.edu/tid/fru38h10
See also http://dida.library.ucsf.edu/tid/dru38h10 and http://dida.library.ucsf.edu/tid/cru38h10
http://dida.library.ucsf.edu/tid/dru38h10
http://dida.library.ucsf.edu/tid/cru38h10
By April 1998 Ryan appears to have had both the acute phase and continuation data
http://dida.library.ucsf.edu/tid/xou38h10
The 1998 Wagner Poster that lists the withdrawal % for the paxil group
WagnerPoster.pdf at the Healthy Skepticism Site
This was an SKB produced poster (I apologize, I mistakenly labeled this in an earlier comment as being from 2000)
http://dida.library.ucsf.edu/tid/dvu38h10
Look at Table 5.
There are 9 adolescents (with identifiers) in the Paxil arm listed as hospitalized or overdose in the acute phase alone. 2 similar events in the imipramine arm and 2 similar events in the placebo arm.
Klein’s Query to McCafferty about possible discrepancies
http://dida.library.ucsf.edu/tid/lpu38h10
A number of these links were included previously but I’m trying to put them in a different order right now if your focus is on trying to identify the individual patients and their adverse events.
This SKB response to the FDA, see pages 3-4
http://dida.library.ucsf.edu/tid/auu38h10
Then there is the correspondence involving Keller, Ryan, Strober, Wagner, and Emslie regarding GSK’s apparent plan to publish a review paper regarding the safety data from the 3 trials, including 329.
Healthy Skepticism Study 329 site
20040204RyantoKellerStrober.pdf
040514KellertoCarp.pdf
20040413KellertoRyanetal.pdf
Keller states in the June 2004 to Ryan, Strober, Emslie, and Wagner:
“we are not without leverage if we are dissatisfied and want to play a little hardball of our own.”
“if we want to fight that battle we will have to go to senior management. I am willing to do that if we agree that after the changes are made to this draft we are still hung out to dry.”
“so that it is 100% clear in this paper that there is no way to read it and think that 329 is being criticized and that it was not written with complete integrity and accuracy given the data we had and should have had as investigators, on the part of investigators and our colleagues from SK who worked on it.”
“otherwise we would look foolish, naive, incompetent, or “biased” (the most likely accusation that will be made) to present things in a way that was favorable to SK, disregarding our responsibility to the proper scientific method, to the public, children and their families.”
After Keller discussed the possibility of going to more senior management in October 1999 about SKB McCafferty’s holding up the 329 JAACAP manuscript, in November 1999 the manuscript was submitted.
In April 2004 GSK’s Carpenter is stating “there is a corporate need/obligation to communicate the key safety and efficacy data from all of these studies to the medical community in a manner which is consistent with the newly revised labeling ASAP. Consequently we have drafted a “review” manuscript which we hope to submit for publication very soon (attached below for your review). This paper presents the key efficacy and safety data for each of the studies individuallyl and it also presents the results of analyses of the pooled dataset (all three studies combined).”
In June 2004 Keller then discussed possibly going to senior management if “dissatisfied.” This apparently out of concern that they could be viewed as “disregarding our responsibility to the proper scientific method, to the public, children and their families.”
My best guess is that the review paper turned out to be this one that came out in 2006: 16553530 in pubmed. I’m not sure how one would get access, but if you can it seems like it would be worth looking at the data they would present.
The available documentation doesn’t really allow us to know what that review would have looked like if GSK had not received Keller et al’s feedback.
I am in total agreement that there needs to be greater data transparency. I am suggesting that after you parse the data more fully you consider taking a page from Allen Frances’ playbook. He realized that addressing himself to the APA was a losing proposition so he went to a broader audience to present a consistent drumbeat regarding poor science, secrecy, and hubris. Most importantly, he tried to address the DSM V’s target audience, its customers if you will. There are many more non-psychiatric users of the DSM than psychiatry end-users.
There are let’s say about 8000 practicing child psychiatrists in the US. There are about 60,000 pediatricians and about 100,000 family practitioners (I’m not clear if these numbers even include other clinicians with prescribing privileges such as NPs). At the very least we are talking a 20 fold difference.
That is why SKB went after JAMA first. That is why the APA is paying attention to Frances (to the extent they are at all).
Parse the raw data. I would strongly recommend that you focus on the safety data. Because overselling efficacy isn’t a shock to many people. JAMA reviewer #2 highlighted, however, the obligation to look out for the primary care provider and give them the data they need to make informed decisions.
It does not appear that Drs. Ryan,Wager, Dulcan, and of course Keller, believed that those physicians could be trusted to make informed decisions if presented the raw adverse reaction data forthrightly. Whatever in the long run turns out to be the correct way of conceptualizing the possible side effects of paroxetine. At the very least you’d know what to look out for and counsel around, since it’s very hard to look at that data and not think that there is at the very least some greater propensity for psychiatric side effects. Don’t tell me about the possible side effects I should look for because I might be too concerned to prescribe? Because that wouldn’t be “responsible”? I think (hope?) that that would resonate with primary care physicians. And if primary care physicians start being wary of JAACAP/AACAP based practice recommendations, that will be something that would likely trigger an engagement in a much more genuine discussion about the issues we are discussing in this small back room of cyberspace. Truly, no offense whatsoever meant to 1BOM. It’s just that in part I suspect you are preaching to the converted.
It would hopefully also be hard for them to read Keller’s comments and imagine that they were discussing using “hardball” with GSK to influence GSK to act as much as possible in the best interest of children and their families and the physicians relying on the resultant information to try to manage their treatment.
Put everything together and see if there is any forum in the journal Pediatrics that would let you present it? I know I am being naive.
Maybe those physicians would begin to wonder if the current system of data ownership and publication, particularly as practiced within child psychiatry is best serving their needs. And how much stock they wish to place in the next set of recommendations endorsed by the handful of primary individuals involved in Study 329.
I hope you are successful in bringing attention to the lack of access to raw data (particularly safety data).
Here is a tip: when you access the UCSF/POGO archive, and this is the only way you can start to truly explore the raw data on the adverse events, keep in mind that you can search the archive to find pdfs (and then within the pdfs themselves) using the unique patient identification numbers. For example 329.005.00011 or can also show up sometimes as 0011.
I don’t have any further information to offer, but I do wish the best of luck to you.
Thank you for your efforts. I hope you stay with the Study 329 subject at a while longer.
Minutiae.
One last thought about the likely receptiveness of AACAP itself to your message, not meant to detract from the main points above:
Mina K. Dulcan, M.D., editor extraordinaire, 1997-2007.
Hendren, Robert; Anthony, Virginia Q.
Journal of the American Academy of Child & Adolescent Psychiatry, Vol 46(12), Dec 2007, 1527. doi: 10.1097/chi.0b013e31815cf53f
The American Academy of Child and Adolescent Psychiatry (AACAP) has been very fortunate to have Mina K. Dulcan, M.D., serve as Editor of the Journal of the American Academy of Child and Adolescent Psychiatry (JAACAP) for the past 10 years. She has served during a period of increased public demand for information and increased scrutiny of our specialty by the media. Also, she has been the pathfinder, steward, and trail maker for the science of child and adolescent psychiatry during a period of rapid growth and great change. She was respectful of authors, nurturing of scientists, while maintaining a standard not to publish similar work over and over again. She made the “trains run on time,” emphasizing promptness. She was diligent and discerning as well as innovative. Her decisions and leadership were always thoughtful and measured. She chose her battles well, stood her ground, and let the science shine through
http://psycnet.apa.org/psycinfo/2007-18374-001
“The officers determine policy and planning (with staff input and expertise when appropriate) and the staff implements. The staff report to the executive director NOT to the members or officers. Ginger knows the issues and is secure enough to address leadership when necessary for the sake of the AACAP and its mission.”
http://www.aacap.org/galleries/aacap_news/virginia_anthony_exec_director_of_aacap_retires_after_39_years.pdf
http://aacap.org/cs/life_members/an_open_letter_to_virginia_q_anthony
http://www.aacap.org/cs/root/…/understanding_how_aacap_works
(note make-up of the executive committee)