per·sev·er·ate (p r-s r-s v v  -r -r t t ) ) |
|
| [verb] | |
| Repeat or prolong an action, thought, or utterance after the stimulus that prompted it has ceased. | |
I hate to perseverate, because it’s a sign of dementia and at my age, you can’t be too careful. But I think Nancy and Alto have happened onto something. Knowing that I think Dr. Trivedi of UT Southwestern is a hole in the academic research landscape into which the NIMH throws money, she sent a link to an infomercial in a West Virginia paper that popped up on a Google Alert [see an outrageous waste…, the article…]. Alto found it in some other papers and suspected TownNews.com which Jamzo ran down for us. So I put it into Google, and discovered that the article blankets the small town world of the whole country! It’s not in our weekly in the Georgia Mountains, but it’s in a paper one county south of us. They’re in a Health and Wellness section of these small papers. None that I clicked on say advertisement.
So I’m not perseverating after all. Forest Laboratory‘s Viibryd® is the one perseverating its way across the country in the small town newspapers. If you don’t know Viibryd®, it’s worth taking a look. I summarized the data when it came to market in 2011 [Viibryd I…, Viibryd II…]. Merck patented it, but it failed two Phase II Trials. Then GSK picked it up and it flunked three Phase II Trials for them. Finally, Forest acquired it and it passed two Phase III Trials barely [or should I say Trivedi-style?]:
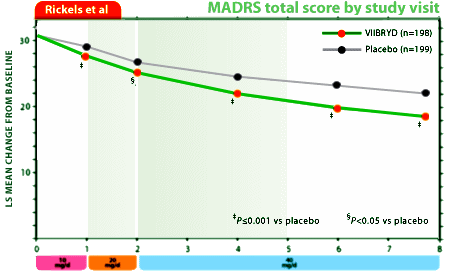
FDA Slams Viibryd: Better Sexual Profile Claim “Not Supported by the Data”
The Carlat Psychiatry Blog
by Danny Carlat
October 26, 2011The September 2011 issue of the Journal of Clinical Psychiatry created history in two ways. First, the journal published this article written by FDA staff critically reviewing the efficacy and safety data of Viibryd, a new antidepressant that the same FDA staff had just approved. Second, it was not just any journal that published the article, but the Journal of Clinical Psychiatry. Why is this so historic? Anybody who has followed this blog over the years has noticed a certain…shall we say, skepticism…toward many of the articles published in JCP, especially those published in its industry-funded supplements. But in this case I’m happy to give the journal kudos for having the courage to publish an article critical of an antidepressant made by a company that pays for drug ads.
Not that any of this information is truly new. Back in April of 2011, my own Carlat Psychiatry Report actually scooped JCP on this issue, when Jim Phelps and I reported in this article that Viibryd-funded authors had tweaked sexual side effect data to make the drug appear “cleaner” than its SSRI competitors. [Some of this tweaking, interestingly enough, occurred in the pages of the Journal of Clinical Psychiatry]. In fact, as we reported and as this new paper elaborates, the studies proved nothing at all about Viibryd’s side effects, because they artfully omitted a crucial element—the inclusion of a comparator SSRI known to cause sexual dysfunction.
The other claim put to bed by the FDA is the idea that Viibryd is quicker to work than other antidepressants. This was based on one of Forest’s trials which showed that Viibryd separated from placebo by week one. The FDA’s article pointed out two problems. First, this data was from Trial 04, which was only one of two large trials submitted as part of the new drug application. In the other trial, Trial 07, Viibryd did not separate from placebo until week 4. Second, neither trial compared Viibryd with an already approved SSRI, meaning that no statements can be made about the new drug working faster than any other drug.
It’s nice to see the FDA stepping up to the plate and proactively publishing articles that dispel company-spread rumors about drug effectiveness. And it’s also great that a usually industry-friendly journal like JCP would publish this.
Earlier in this century, when I was looking at a lot of different websites with discussion groups where psyche meds were being discussed, I accidentally discovered the viral advertising of a drug that had not survived the first round in human testing. I asked my psychiatrist about a med that was supposed to help with night terrors that I had read rave reviews of. He told me that testing was stopped because it caused necrotizing fasciitis. Mistaking my expression for a lack of comprehension, he told me what necrotizing fasciitis was. I knew what it was, but felt a certain dumbstruck awe that meds were being pedaled virally before testing had been done for the most basic safety. The depth to which some will stoop to be rat b***ards gambling with peoples’ lives for many may never cease to amaze me.
gambling with people’s lives for money
Hello, brain-fog.
There’s a paper somewhere that describes this particular p.r. strategy: Offering small newspapers and shoppers “free” editorial that’s barely disguised advertising.
I wouldn’t be a bit surprised if TownNews.com has some kind of remunerated relationship with Forest Labs’s p.r. people, and acts as distributor of this stuff. It’s probably part of their business model. It’s up to editors to decide to run it. They get press releases all the time; the lazy ones run them unedited.
I suggest someone with an MD write to Trivedi and tell him he’s made a fool of himself.
http://profiles.utsouthwestern.edu/profile/17410/madhukar-trivedi.html