 Dr. Roy Poses of Healthcare Renewal often writes about a concept – the anechoic effect [see themes…]. We all know about it. Some big story comes along and there’s a big reaction, outrage around, but then interest peters out and it’s forgotten – worse, nothing is done about it. It happens all the time. And in keeping up with the antics of the pharmaceutical companies, it’s the rule rather than the exception. There ought to be a registry of things to keep on the front burner. In my case, the registry is some phrases scratched on the back of a coffe stained envelope pinned to the wall. One of them says "Plumlee – Zoloft?" Back in the beginning of 2013, I ran across a suit, Laura A. Plumlee et. al. v. Pfizer [see a wide net…], that was intriguing. It alleged that Zoloft didn’t work and asked that Pfizer refund the money to those taken in by the drug’s ads. At first, it seemed far fetched, but not after I read the case. So I went looking for the NDA on the FDA site, but it wasn’t there. So I submitted an FOIA request to the FDA and when it showed up, it gave me plenty to write about:
Dr. Roy Poses of Healthcare Renewal often writes about a concept – the anechoic effect [see themes…]. We all know about it. Some big story comes along and there’s a big reaction, outrage around, but then interest peters out and it’s forgotten – worse, nothing is done about it. It happens all the time. And in keeping up with the antics of the pharmaceutical companies, it’s the rule rather than the exception. There ought to be a registry of things to keep on the front burner. In my case, the registry is some phrases scratched on the back of a coffe stained envelope pinned to the wall. One of them says "Plumlee – Zoloft?" Back in the beginning of 2013, I ran across a suit, Laura A. Plumlee et. al. v. Pfizer [see a wide net…], that was intriguing. It alleged that Zoloft didn’t work and asked that Pfizer refund the money to those taken in by the drug’s ads. At first, it seemed far fetched, but not after I read the case. So I went looking for the NDA on the FDA site, but it wasn’t there. So I submitted an FOIA request to the FDA and when it showed up, it gave me plenty to write about:- zoloft: the approval I…
- zoloft: the approval II…
- zoloft: the approval III…
- zoloft: beyond the approval I…
- zoloft: beyond the approval II…
- zoloft: the epilogue…
… - graphic reflections…
They submitted six studies [only one made the grade, and it was a very weak showing]:
| Placebo Controlled Clinical Trials
|
||||||
| Study
|
Site
|
Type
|
Outcome
|
|||
| protocol 103 |
outpatient | fixed dose | questionable | |||
| protocol 101 |
inpatient | fixed dose |
negative | |||
| protocol 310 |
inpatient | fixed dose |
negative | |||
| protocol 104 |
outpatient | titrated dose |
positive | |||
| protocol 315 |
outpatient | titrated dose |
negative | |||
| protocol 320 |
outpatient | open label, relapse | whatever | |||
The FDA reviewers did not recommend approval. The committee was on its way to denying approval when the head of the FDA [Dr. Paul Leber] who had assured Pfizer he could get it approved entered the discussion with a speech and, as he predicted, he got "it through." It was hardly an exemplary day for the FDA. Reading it, I could easily see why the suit was filed. The plaintiff was right on target. Oh. by the way – it had been turned down already in Europe, but the FDA committee didn’t know that [because Pfizer didn’t tell them].
This graph is number of prescriptions written by year. Zoloft passed Prozac in 2000 [about halfway through its patent life], and it continued to dominate the market share until going generic in 2006 [when it was replaced at the top by generic Zoloft]. It was a $30 B drug: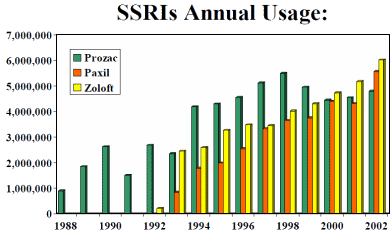
Lawyers and Settlementsby Gordon GibbMarch 24, 2014A proposed Zoloft class-action lawsuit alleging Zoloft is a defective drug because it offers little more efficacy than a placebo, or so it is alleged, was recently tossed by a federal judge due to a time-barring issue and other legal implications. However all is not lost; the presiding magistrate left the door open a crack for a possible continuation of the complaint, with some revisions.
In Plumlee v. Pfizer Inc., Case No. 5:13-cv-00414, in the US District Court for the Northern District of California, plaintiff Laura Plumlee took Zoloft manufacturer Pfizer to task for marketing a drug that was alleged to be ineffective, with questionable efficacy, due to a claim that most clinical trials found that Zoloft was no more effective than a placebo, or so Plumlee claimed. Her lawsuit alleges that Pfizer purposely omitted, in Zoloft labeling, any studies that showed Zoloft to be ineffective, while favoring studies that showed Zoloft was, indeed, more effective than a placebo. Plumlee also alleged that Pfizer’s marketing and advertising was also misleading in touting Zoloft, an antidepressant, as effective.
However, Plumlee’s claim was dismissed not on her argument of effectiveness, but due to time barring. It has been reported that Plumlee brought her defective drug lawsuit under two statutes observed by the state of California: that of the Unfair Competition Law, and the Consumer Legal Remedies Act and False Advertising Law.
Was plaintiff’s claim time-barred?
The two aforementioned statutes, under California law, carry limitations of four years and three years, respectively. In her ruling dismissing the plaintiff’s claim, US District Judge Lucy Koh ruled that Plumlee’s complaint went beyond the limitation boundaries, given the plaintiff’s claim that she last used Zoloft in 2008 but waited until January 2013 to bring her lawsuit.lumlee challenged that such limitations were tolled until 2012, the point at which Plumlee first discovered that Zoloft had been misrepresented. The judge, however, held that Plumlee’s claim to discovering Zoloft’s inadequacies in “early 2012” was too general a frame of time. Judge Koh also was not satisfied with the detail supporting the time and surrounding circumstances of her discovery.
To that end, the judge pointed to the existence of various scientific articles – cited by the plaintiff – that had been published long before Plumlee brought her drug defects lawsuit, and thus did not accept the plaintiff’s claim. However, the judge left the door open.
All is not lost for this Zoloft defective medical products action
In dismissing the plaintiff’s claim, Judge Koh is allowing Plumlee to amend her complaint going forward. It is telling, as well, that the California judge ruled that Pfizer has the freedom to access certain aspects of the plaintiff’s medical history. Plumlee had sought to block Pfizer’s access to her medical records. A previous magistrate’s ruling that allowed Pfizer access was supported by Judge Koh on grounds that Plumlee had waived any privilege of protecting her medical history when she argued that the statutes of limitations were tolled due to her learning of Zoloft’s alleged deficiencies only in early 2012.Plumlee, according to various reports, had sought to represent a proposed class of plaintiffs who may have used Zoloft from the point at which it was introduced to market in 1991, through to present day. However, the judge suggested that Plumlee may not be typical of the class, given that she claims to have used Zoloft for a period of three years even though it did not appear to be working for her. Records also demonstrated that the lead plaintiff relied more upon Zoloft marketing and advertising, than the advice of her doctor.
Pundits suggest that in leaving the door open, the judge feels the proposed class-action lawsuit may have merit, in spite of deficiencies exhibited by Plumlee’s claim. The potential, thus, is for Plumlee to amend her claim that satisfies time-barred limitations and other deficiencies as articulated by the presiding judge. Could the proposed class-action lawsuit proceed with a different lead plaintiff?
Harmful drugs are often shown to carry risks, in spite of the position of the US Food and Drug Administration (FDA) that holds that a drug’s benefits outweigh the risks for the class or constituency of patients to which the drug is targeted. In the same vein, however, drug defects can also include deficiencies that suggest a drug is not worth the financial outlay, either by an individual or group, in exchange for potentially limited effectiveness.
The aforementioned Zoloft lawsuit alleges Zoloft does not live up to its promises. The proposed class action, alleging defective medical products (Zoloft, as ineffective), could continue with amendments – but perhaps not in its present form.
Law360By Sindhu SundarSeptember 02, 2014Pfizer Inc. on Friday defeated for the second time allegations that it greatly exaggerated the efficacy of its antidepressant Zoloft, when a federal judge in California ruled the proposed class action claims are time-barred and dismissed the suit with prejudice.
U.S. District Judge Lucy H. Koh, who in February had dismissed Laura Plumlee’s suit but allowed the plaintiff to amend her suit to address the court’s timeliness concerns, granted Pfizer’s motion to dismiss the suit with prejudice Friday…
Plumlee last bought Zoloft or its generic equivalent in 2008, and by the time she brought her suit in 2013, she had exceeded by at least seven months the statutes of limitations under the various California consumer protection laws she invoked, Judge Koh ruled.
"The court finds that each of plaintiff’s claims is time-barred and that despite being granted an opportunity to amend her complaint, plaintiff has still not met her burden of showing that the statutes of limitations have been tolled by the delayed discovery rule," Judge Koh said in her opinion.
Plumlee, who had filed her original suit in January 2013, claimed that she did not learn about Pfizer’s alleged over-representation’s about Zoloft’s effectiveness until she watched a "60 Minutes" segment in May 2012, according to the order.
"We are pleased with the decision and believe the court applied California law correctly in ruling to dismiss the case with prejudice," Pfizer spokesman Steven Danehy said in a statement Tuesday. "Pfizer has always believed that the plaintiff’s amended complaint fails to adequately address the deficiencies of the original complaint, which was previously dismissed."
An attorney for Plumlee could not immediately be reached for comment Tuesday.
Plumlee had sought to represent a proposed class of patients who used Zoloft made by Pfizer between the drug’s launch date in 1991 through the present. She claimed that Zoloft’s labeling failed to mention the studies showing it to be ineffective, that Pfizer favored researchers who showed Zoloft to be effective, and that the company’s advertisements misleadingly touted the drug as effective, among other allegations.
She claimed for instance, that Pfizer buttered up doctors with blandishments including ski trips and "fancy" meals, to encourage them to prescribe Zoloft, according to court documents.
Well I hope somebody passes this reference along to the trial lawyers:
1: Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R,
Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009 Feb 28;373(9665):746-58.
And this quote from the authors in their subsequent response:
“Sertraline has the best overall profile and the most robust data.”
Too bad that very few patients have the resources to use the tort tool to nail pharma’s hide to the wall for misrepresentation of psychiatric drugs.
From whence will justice come? These misrepresentations will be recycled over and over into clinical practice for generations.
Since, the field argues that the risks of suicide is the most compelling reason to prescribe antidepressants, shouldn’t an antidepressant that is ineffective be considered a risk factor for suicide by that logic?
Reads like the lame suing the shame. The plaintiff won’t release her medical records in a suit claiming ineffectiveness, as per what precedent? Pfizer can’t challenge her chronic hypothyroidism or diabetes known tied into treatment resistant depression scenarios (I don’t know her hx, but could be legit issues?).
Oh, and Pfizer is suppressing data, just like Lilly, Glaxo, Forrest, Azta Zeneca, etc. why not time for all outraged psych patients just take a class action suit against all of Big Pharma for, what, 100 billion in damages?
What about the psychologist claiming military psych docs are increasing suicide rates in soldiers by treating them with antidepressants? See http://www.cbsnews.com for that story?
Meds aren’t for everyone, but, this ongoing argument that meds are for no one, who is running this debate? Characterological disordered people in psychiatry and the public. Sheesh, if it ain’t the crap dialogue in DC, then we have to read it at sites like this about health care??
When you all laid silent with managed care sweeping thru America sort of how ISIS now is running rampant in the Middle East, just because no doctors were literally beheaded, it was OK to be figuratively emasculated then?
Think about it.
Spending 1.5 billion to get a drug approved and then getting sued for lack of efficacy isn’t technically double jeopardy, but to me its kind of the same idea. If we establish a habit of suing for lack of efficacy that’s going be a lot of misallocation of resources away from progress and into trial attorneys’ bank accounts. If you want an example of the kind of injustice this can lead to look at the silicone breast implant cases of the nineties.
Primum non nocere also applies to medical policy…I don’t want to cheer class action attorneys.
There needs to be an effective but especially cost effective solution. I say can the Phase 3 trials in exchange for complete data transparency as suggested in some of these articles.
Cheering on class action lawsuits is not the solution. Even if we don’t like the defendant.
Dr. O’Brien, would you feel differently about suing if god forbid you had prescribed Paxil to kids younger than 18 on the basis of the fraudulent 329 study that you thought was understandable legitimate at the time and had your reputation smeared because your patient suffered adverse affects? Just trying to determine if you feel suits are ever justified.
Personally, I don’t think lawsuits should be taken lightly. But many times, they seem to be the only way for things to change.
And reiterate Alto’s point, when will the justice come?
I like the idea of “pinning the registry of forgotten big cases to the wall”. I would like to include the fact that Medicare cannot bargain for drug prices. At the moment that seems to be one of the biggest financial absurdities of capitalist thinking.
That list might quickly get too long for coffee stained envelopes.
I don’t suits are never justified and I was specifically talking about efficacy lawsuits. And my point was that having to go through both approval and unpredictable efficacy lawsuits is too big of a burden. One or the other but not both. And let comparison efficacy be sorted out in doctors’ offices and clinics.
As much as we me dislike Glaxo or Pfizer for some of these shenanigans, in the big picture they have save lives and improved health. That cannot be said about John Edwards.
Always remember there is no chance that aspirin would get through the FDA today and no chance the manufacturer wouldn’t be sued if it were a new drug.
I think we need to radically rethink the whole process.
Sorry about the grammar in the last post. “I don’t think suits” is how it should have started.
Sunshine is the best disinfectant and the only one that is effective before the damage is done. I would even have the grant process published on the Internet. And all raw data public with a must publish stipulation to any grant. Basically make it impossible to spike data or studies.
This blog and blogs like it will do more to clean up KOL-pharma bias than any attorney.
Getting attorneys or regulators involved late in the game is catharsis but not a solution. Government is not a solution. Nemeroff and Schatzberg continue with business as usual. Serzone was one of my favorite clinic antidepressants and the government foolishly went beserk on a side effect I felt perfectly capable of monitoring. We have a system that is neither effective nor inexpensive. I don’t want any more misallocation of capital. Do we want to solve the problem or simply engage in moral vanity and grandstanding?
Yes I know the company pulled it but it was under government pressure because of 1/250K patient years hepatotoxicity.
I really need to post after the first cup of coffee when the cobwebs have cleared.
Dr. O’Brien, how would you close the feedback loop when it comes to patients injured by misrepresented drugs?
Being able to parse data from drug trials is crucial as an honesty check (which is clearly needed in the pharmaceutical world). But that efficacy and safety data is largely theoretical anyway.
The real-world experience of large numbers of patients post-marketing is extremely important to make the risk-benefit evaluation more accurate for any individual patient and to capture patterns of error in clinical practice.
However, national collection and analysis of post-marketing data has been systematically stymied by commercial interests. In the U.S., the tort system is the only way to bring patient injury and clinical error to light.
You might say post-marketing surveillance has been privatized in the lawsuit system. (And in online patient complaints, but no one takes them seriously but the patients.)
So what is your alternative? Feedback about patterns of injury in clinical practice could only improve the profession, wouldn’t it?
A sunshine law as I explained. The original sin to most of these problems is secrecy. Would have prevented these issues from ever going to litigation in the first place. The fact that the FDA has not insisted on this years ago is further proof of their incompetence.
The existence of this blog proves that torts are not the only way to bring these situations to light. Torts cause more problems as they solve and are a cleanup after the mess has already occurred. Class actions are often won on junk science and emotion as the silicone breast implant case illustrates.
Let me also add this observation…if we’re going to start advocating efficacy lawsuits, then 98% of nutritional stores and alternative medicine clinics would be sued out of existence…and quite a lot of psychotherapy practices. There are plenty of experts who also believe that the talking cure is nothing more than a relationship effect and they have their studies backing their viewpoint. I happen not to agree but that won’t stop the lawsuits from being filed.
Be careful what you wish for, you might get it.
A sunshine law will do absolutely nothing to collect and analyze post-marketing issues. Lawsuits at least bring cases of injuries to light.
The original article was about lawsuits over efficacies, not side effects that were hidden from regulators.
One principle of tort law is there has to be damages. What are the damages? That the medication trial stopped them from going on the SSRI that’s 90% effective instead…namely fairydustapene?
Everyone here raise their hands if they’ve ever given a treatment in which the effectiveness has ever been questioned. That includes psychotherapy or the prescription of any SSRI/SNRI. Not to mention mood stabilizers or TCMS. I thought so. The only thing not on the list really is ECT and that’s a whole different legal mine field for different reasons. Now think this through and realize you ultimately cheering on your own demise.
Solutions, not catharsis.
Hence my earlier comment, the lame sue the shame. Too many who shriek for consequences with psychotropics either deflect or minimize when we note similar patterns or percentage side effects with other medication groups, like, do we remove penicillins because 10% of the population have allergic reactions, or that 20+% in cancer chemotherapy/ radiation have debilitating reactions.
No, the bias is very transparent here, it is only about psychiatry, and irregardless if even 50% achieve substantial reduction of psychiatric impairment, if not remission, those 10% who have poor reactions outweigh the needs of the many.
Big Pharma is a screw up, but, what, go back to Elavil and Thorazine for depression and psychosis? Focus on the bigger picture, and that is who needs meds, and who doesn’t. But, that does not fit the narrative of abolitionists, eh?
If scientifically and mathematically illiterate juries instead of doctors get to decide efficacy, and therefore what gets prescribed by legal precedent, medicine will enter the dark ages.
These are debates that need to be settled in case conferences, grand rounds, and blogs like this, but not in courtrooms. And not under the don’t offend anyone’s feelings ever rules of the Psychiatric Times or DSM committees (low on conscientiousness, high on agreeableness), but the spirit of scientific inquiry advocated by Meehl:
http://www.tc.umn.edu/~pemeehl/099CaseConferences.pdf
James, thanks for the link to Paul Meehl… what a refreshing curmudgeon!
I highly recommend “A Paul Meehl Reader.” It includes a lecture by him on a DVD. The guy was a genius of quant psychology.
Dr. Hassman, I believe Dr. Ben Goldacre has mentioned that generally, the data on pharmaceuticals is so unreliable that he feels he can’t prescribe meds on a safe basis. So for me, the issue is the trials on meds in general and whether pharmaceutical companies deliberately cover up problems or not.
Of course, intent isn’t always easy to determine and I do agree that there can be side effects with some meds that people had no way of being able to tell ahead of time. But when there is a deliberate attempt to cover up things that end up injuring patients, they should have every right to lodge a lawsuit.
Dr. O’Brien, with all due respect, I find it insulting to imply that non medical professionals are incapable of reading scientific data to make a fair judgement in a trial. I certainly wouldn’t belong on a jury but I feel folks like Alto and Wiley could do a great job. By the way, I know you didn’t intend to come across that way.
Additionally, when patients have depended on doctors to make things right, they waited a long time. Many years ago, there was a horrific case of malpractice by a psychiatrist in my area that resulted in the death of someone due to extreme excessive drug prescribing. Several doctors realized what was going on but were too scared to say anything out of fear they would suffer repercussions. It took a very long time for justice to be obtained for the family of the surviving victims.
Another example is the kids in foster homes who are the victims of excessive drug prescribing. I don’t hear any of your colleagues speaking out against that.
It was a comment on the general state of scientific education today. I’m sure Alto and Wiley would be fine jurors but they are not representative of the public’s limited understanding of science. You know, of course, that in a medimal trial voir dire, the last thing the plaintiff’s attorney wants is someone with a degree in science. What they want is someone angry, antiauthoritarian and easily manipulated. Jury consultants will tell them no engineers, please. And God forbid, no doctors.
Statements like “the science is settled” are accepted by the public as scientific authority. Eyes glaze over when statistics are discussed.
An informed public would mock whoever tried to get away with such a claim.
The more important point is that you don’t want nonmedical people deciding efficacy for doctors. Much of the lay public believes, without a shred of evidence, that alternative medicine is just as valid as most Western medicine. And they watch shows like Dr. Oz. The lay public for emotional reasons could determine raspberry ketones are efficacious but not ECT.
I think many of you are getting confused with the very important difference between efficacy torts and malpractice torts involving damage. Your example does not relate at all to the point I am making.
If you want to decide efficacy (not damages, for the fifth time, please), in the courtroom, then many things in medicine are fair game. That would include back surgery, knee meniscectomies, low fat diets, and in the view of some, statins and the like. Acupuncture and chiropractic medicine would be right in the bullseye. In addition to most of psychiatry as I pointed out.
Ironically, Scientology would weather the storm completely unscathed as they are now a religion and not subject to these burdens. So the E-Meter is in no danger at all.
Physicians should be held to accountability when true harm has occurred, so I am not saying people should not sue for malfeasance, incompetence, or reckless carelessness. That said, this lawsuit above is at least a bit nefarious when the plaintiff thinks she can hide her medical history and claim the treatment was inappropriate. The broad brush that “all psychotropics” are inappropriate and nefarious, well, just doesn’t cut it as a precedent. As I said above earlier, not all patients should be on meds, but, neither should meds be denied all patients. We need to stop arguing in absolutes.
Again, it is time for colleagues to out the inept and criminal element in our profession, dump them on the street, or at least find creative and effective ways to ostracize and denounce with effectiveness their agenda of selfishness and greed. And start with most in the APA.
Also, again, as I wrote in one of my posts months ago, one question I feel a new patient should ask a psychiatrist at the time of the first visit is simply this: Do you belong to the APA? Prospective patients should learn that the APA is not an altruistic, caring organization as a whole, so at least follow up with “why do you belong” if the doc says “yes I belong to the APA”.
(I offer the post with some reticence as I don’t promote myself, but, readers should understand the perspective I am noting above:
http://cantmedicatelife.com/2014/02/01/seven-questions-a-patient-should-ask-of-a-psychiatrist-when-beginning-treatment/ )
I have yet to have a colleague who says in one breath ” I care about patients and the direction of psychiatry”, who simultaneously still belongs to the APA, reasonably explain how the two are compatible, with what we know the APA really is doing to psychiatry. Maybe patients should decide if that attitude fits for them in seeking out providers as well.
Hey, when you rely on the courts to decide what is right for a health care matter, good luck with that being responsible and effective over 80% of the time. In fact, judges by in large actually are biased and non objective to mental health matters, and if they could, would try to rule to hurt both the doc and patient.
Don’t believe me, talk to people who have had their mental health issues ruled on in courtrooms. they won’t tell you much of positive and glowing experiences, I can almost guarantee that!
The Plumlee lawsuit was not an academic discussion of efficacy. It was a post-marketing injury suit. The basis of the injury is lack of efficacy. How the plaintiff was going to prove damages, I don’t know — that’s up to the plaintiff’s legal team.
As for other suits based on lack of efficacy — if you, for example, were treated with an antibiotic for an eye infection, lost the eye, and subsequently found the drug manufacturer had lied lied lied about efficacy to get the drug approved by the FDA, would you feel you had a beef with the drug manufacturer?
Perhaps would you shrug and say “That’s the free market for yah. Buyer beware. That pharmaceutical company does a lot of good.”
So what would your corrective be for such post-marketing injury, if you don’t like the delegation of that to the tort industry?
Basic principle of tort law is that someone be made whole for damages. Efficacy lawsuits are not based on that. The damages in an efficacy claim are basically the costs of treatment. Which is pretty much small claims. The antibiotic analogy fails because there are other antibiotics that are 95% effective. The only antidepressant that qualifies is fairydustapene. Also, in the case of antibiotic trials, it would be impossible to fool the FDA because there are clear objective findings. The infection doesn’t get better. So what you are describing couldn’t happen and has never happened…an antibiotic with zero efficacy getting through the process.
And your argument is also completely wrong on my sunshine law. Had it been in effect, there is no way the manufacturer would have continued making that claim. The competitors would be all over it.
There are many things in medicine in which efficacy and comparative efficacy are debated. In fact most of it. Be careful what you are cheering for. Unless you are a trial attorney, then it’s just a big windfall.
Come to think of it I have a better idea that efficacy lawsuits. Let’s just have a tax of 3000 per year on every family in America and hand it over to plaintiff’s attorneys. It will be the same result without all the unnecessary drama. And there will still be some treatment options left when patients get sick.
Interesting. My analogy fails because unlike antibiotics, the efficacy of which are difficult to fake, it’s easy to fake the efficacy of a drug for a condition with no physical markers. I’d have to agree with you there.
Yes, I guess if the drug companies could debunk each others’ research, that would theoretically keep them honest.
But I have never seen a pharma-sponsored paper taking apart a study performed by another company, have you? Many of these studies have clear design flaws that could easily be critiqued.
Instead, drug companies compete by generating their own rigged studies for their own products.
Must be a gentleman’s agreement in effect. They all want to be free to lie about their products. Halt the lying and part of the industry — the part looking for blockbuster profits from weak drugs — would collapse.
My point was that sunshine law would affect only registered studies (whatever that means). It would have nothing to do with post-marketing patient experience, which is contained mostly in anecdotal reports by patients and, occasionally, doctors, plus the rare lawsuit that gets public attention.
Studies done for FDA approval and dosing are only the theoretical beginning of understanding a drug’s risk-benefit ratio. Such studies are only approximate in assessing efficacy and notoriously underpowered in capturing risk. The real proof is in the post-marketing data.
Currently in the US there is no mechanism capturing this post-marketing information. As I said above, the corrective function has been privatized by the tort industry. The sampling methods utilized are crude, amounting to whomever the attorneys think has a good case for good money.
This is not a good sampling mechanism but it does get the FDA’s attention, prompting such warnings as for withdrawal on the Paxil label and pregnancy risks on several antidepressant labels.
Isn’t an “efficacy lawsuit” your own construction? I thought the Plumlee tack was ingenious, though it seems it was poorly executed. And it got Dr. Mickey’s attention for several excellent posts in addition to some press, which maybe was its objective anyway — because no other corrective avenue exists.