The topic here is augmentation with Atypical Antipsychotics in treatment resistant depression [once again], but it’s also about meta-analyses in general. In my recent in the land of sometimes[3]…, I was looking at the techniques used in making the forest plots now widely used in meta-analyses. It came to mind as I was comparing these two examples [essentially covering the same ground]:
by J. Craig Nelson, M.D. and George I. Papakostas, M.D.American Journal of Psychiatry. 2009 166:980-991.
Objective: The authors sought to determine by meta-analysis the efficacy and tolerability of adjunctive atypical antipsychotic agents in major depressive disorder.Conclusions: Atypical antipsychotics are effective augmentation agents in major depressive disorder but are associated with an increased risk of discontinuation due to adverse events.
In creative funding III & some other things… where I reviewed part of it. I said, "It’s a decent article, well researched with lots of useful information" but I could’ve added, "but there’s a much more comprehensive one coming." Alto mentioned this next one in the comments, and it answered some questions I hadn’t gotten around to asking:
by Glen I. Spielmans, Margit I. Berman, Eftihia Linardatos, Nicholas Z. Rosenlicht, Angela Perry, and Alexander C. TsaiPLoS Medicine. 2013 10[3]:e1001403.
Background: Atypical antipsychotic medications are widely prescribed for the adjunctive treatment of depression, yet their total risk–benefit profile is not well understood. We thus conducted a systematic review of the efficacy and safety profiles of atypical antipsychotic medications used for the adjunctive treatment of depression.Conclusions: Atypical antipsychotic medications for the adjunctive treatment of depression are efficacious in reducing observer-rated depressive symptoms, but clinicians should interpret these findings cautiously in light of [1] the small-to-moderate-sized benefits, [2] the lack of benefit with regards to quality of life or functional impairment, and [3] the abundant evidence of potential treatment-related harm.
The 2009 paper was essentially a collection of forest plots: Response Rate, Remission Rate, All cause Discontinuation Rate, Discontinuation Rate due to Adverse Events, in several groupings – all using Dichotomous [categorical, yes/no] Outcome Variables. Recall from in the land of sometimes[3]… that with categorical variables, the criteria used matters, and in this case, the criteria for Remission varied among studies. In addition, categorical variables are derived. Continuous variables are independent of criteria, universally measured, and closer to the raw data. Spielmans et al provided the resulting Hedges g Effect Sizes in a table, so I put them into forest plots. First, here are the clinician rated metrics…
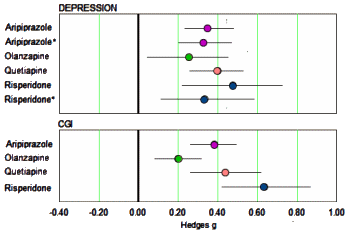
…mirroring the finding in Nelson et al of a moderate effect from the augmentation on Depression [MADRS, HAM-D] and Global Inventory. But Spielmans et al did something else. They looked at the [frequently ignored] self-report measures:
The only continuous self-report measure of depression used in these trials was the Inventory of Depressive Symptomatology Self Report. Continuous measures of quality of life included the Quality of Life Enjoyment and Satisfaction Questionnaire [Q-LES-Q] and the Short Form 36 Health Survey [SF-36]. The only continuous measure of functional impairment employed in these trials was the Sheehan Disability Scale [SDS]…
These self-report measures are often given short shrift in articles. But if you think about it, they should be front and center. When I write a prescription, the next meeting usually starts with a report about the effect of the drug – efficacy and side effects. I recently joked about my patients’ "fingers thing" as an outcome measure [see an aside…], but I was also dead serious.

In the clinical setting, a medicine either helps the patient or it doesn’t, and that’s the whole reason for giving it. So I made a forest plot of the self-report measures Effect Sizes too…
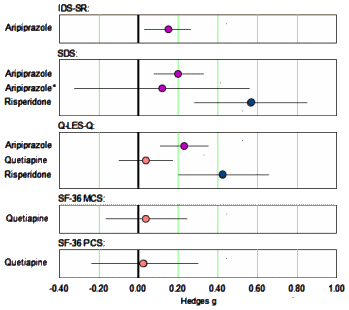
… that was certainly informative. Except for Risperidone, these measures were weak to nil. And they looked further. The asterix indicates the results after removing cases with protocol violations for Ariprazole. But when they looked at the Risperidone study, there was something else:
…The effects of risperidone may have been exaggerated by the reliance on post hoc analysis rather than a priori analysis in the largest study of the drug, as the effect of the drug was greater at 6 wk (g= 0.46) than at the prespecified primary end point of 4 wk [g= 0.32].…The effect of aripiprazole on quality of life/functioning should be interpreted with caution, as the effect for the drug on the SDS was very small and no longer statistically significant when patients who violated study protocol were excluded from analysis [g=0.12, p=0.08]. Similarly, the effect of risperidone on quality of life/functioning should be interpreted tentatively since it is largely driven by post hoc analyses.
One important thing to keep in mind about meta-analyses, for all the clarity they bring by including a range of studies, they also hide the details and context of any given study by simply reporting "the numbers." And this is such a case. Most of the depression data and all of the SDS and Q-LES-Q numbers for Risperidone come from a 2007 Janssen report by Mahmoud et al – an all Janssen employees article with a tainted history [see ANTIPSYCHOTIC DRUGS FOR DEPRESSION?, trembling earth, mirrored water…, racketeer influenced and corrupt organizations…, optional reading…, etc]. This was a darkest hour that involved a 2005 review article on this topic by Dr. Nemeroff [Use of Atypical Antipsychotics in Refractory Depression and Anxiety]; a 2006 article by Rapaport, Keller, Nemeroff, Mahmoud and other Janssen employees initially claiming efficacy for Risperidone augmentation, but later declared negative by the first author [see Effects of risperidone augmentation in patients with treatment-resistant depression: Results of open-label treatment followed by double-blind continuation, Response by Dr. Bernard Carroll, Author’s Reply]; and much much more. So I would say that Spielmans et al’s caution to "interpret… tentatively" the Risperidone data is an understatement at best. They might just as well substitute "ignore altogether."
There’s nothing particularly wrong with the 2009 Nelson and Papakostas meta-analysis. They reported "the numbers" accurately. But we owe a debt of gratitude to Spielmans et al for going the extra mile and looking critically at the nuances of the individual studies involved [many more findings than mentioned here]. They make the risk/benefit equation come alive for the clinicians who actually use these drugs and the patients who take them…
UPDATE: I failed to mention that Spielmans et al also compiled those Adverse Events with significant Effect Sizes [p<0.10] shown below [truncated]:
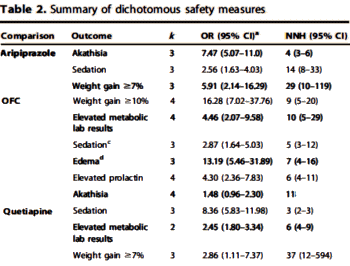
Um, have we ever seen a coherent pharmacologic rationale for using these atypical antipsychotic drugs to treat depression? There has been lots of speculative and self-serving hand waving but nothing that hangs together. And remember, these are nonpsychotic depressed patients.
In my clinical practice, I have rarely seen the need for adding these meds. Since working in Community Mental Health for the past year, I have inherited a panel of non-psychotic patients many of whom are on Abilify and Seroquel. When I explain the risks involved many are willing to be tapered off. When this is done slowly, the drug is never missed. The greatest effect of them that I see is a kind of zombification and metabolic syndrome. The patient will complain less, speak less and the psychiatrists can get through their med checks more efficiently.
Debra,
That was exactly my experience. I had to learn not to come on too strong and to taper very slowly, but I’ve never yet had a patient ask to return to the medication. I thought there might be an anxiety problem afterwards, but it didn’t happen. In fact nothing ‘bad’ happened…
It gets worse I’m afraid. From Richard Lehman of the BMJ:
“I’m naturally depressive, and as I become less mobile, more forgetful, and closer to death, I’m sure to turn into a horribly morose old codger. They will fill me up with antidepressants, but as I continue to codge morosely, they will give me aripiprazole. That will give me akathisia, but never mind. It is never too late for new experiences.”
“In this American trial, people of about my age who were thought by their psychiatrists to have depression were put on maximal doses of venlafaxine for a month and if that didn’t seem to work, they were randomized to get aripiprazole or placebo. A quarter of those in the aripiprazole group developed akathisia and 17% developed Parkinsonism. A greater proportion of participants in the aripiprazole group achieved remission than did those in the placebo group (40 [44%] vs 26 [29%] participants; odds ratio 2•0 [95% CI 1•1–3•7]. This is described in the editorial as a “major leap forward.” Major leaps forward do not have confidence intervals that begin at 1.1. Also, if a quarter of patients given aripiprazole were asked to leap forward, they would probably fall over.”
Here’s the blog, with links to this revolting study:
http://blogs.bmj.com/bmj/2015/12/14/richard-lehmans-journal-review-14-december-2015/