Continued from a worksheet post…
In April 2015, the FDA approved a new Atypical Antipsychotic, Brexpiprazole [Rexulti®]. Around the same time, there were two articles about the Brexpiprazole clinical trials, both released on-line ahead of print in prominent journals [see the spice must flow…]. But there were some odd things about those articles. Each one had only one academic author – the rest were pharmaceutical company employees. But there was more [see anything goes…] – the academic authors were from the same department of psychiatry, the same institute [Feinstein Institute for Medical Research], with similar conflicts of interest, and the medical ghost-writers were also from the same communications firm [QXV Communications, Macclesfield, U.K.]. These were the studies that the FDA evaluated in the approval process. These articles were virtual clones.
Lundbeck Press ReleaseSeptember 24, 2014
H. Lundbeck A/S [Lundbeck] and Otsuka Pharmaceutical Co., Ltd. [Otsuka] today announced that the US Food and Drug Administration [FDA] has determined that the New Drug Application [NDA] for brexpiprazole for monotherapy in adult patients with schizophrenia and for adjunctive treatment of major depressive disorder [MDD] in adult patients is sufficiently complete to allow for a substantive review and the NDA is considered filed as of 9 September 2014 [60 days after submission]. The PDUFA date is July 11, 2015…
by Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, Nyilas M, Carson WH, Sanchez R, and Eriksson H.Journal of Clinical Psychiatry. 2015 76[9]:1232-1240.
by Thase ME, Youakim JM, Skuban A, Hobart M, Augustine C, Zhang P, McQuade RD, Carson WH, Nyilas M, Sanchez R, and Eriksson H.Journal of Clinical Psychiatry. 2015 76[9]:1224-31.

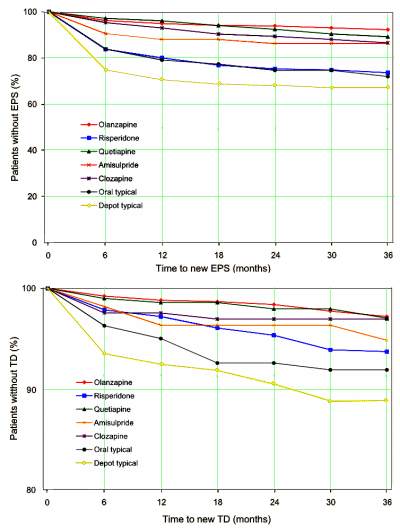
I’m not in love with this story about the marketing practices of big PHARMA, but that’s not really any of my business. But there’s something else that is. When I first started working as a volunteer in the clinics up here, it seemed like everyone was taking Seroquel®. I couldn’t figure out why. At the time, it was the number one selling drug in the country. That’s actually what got me blogging here in the first place. Over time I finally got every non-psychotic patient off of the Seroquel® – worried about Tardive Dyskinesia and the metabolic syndrome, particularly with long term use. I was kind of proud about getting them off that drug. Recently, our clinic has expanded its scope, so there has been an influx of new patients, at least new to me, and guess what? – a lot of them are on Atypical Antipsychotics [not just Seroquel®, but Abilify® and the others]. But here’s the bad part, I’ve got three of those new cases who have definite early Tardive Dyskinesia [a lot for my small practice]. Mercifully, two seem to be waning now they’re off of the drug [fingers crossed], but I’m afraid one is probably around forever. They’ve all been on the drugs for a long time [which is what happens these days]. And I doubt such symptoms happen in short trials.
Although the acute efficacy of the adjunctive atypical antipsychotics is well established, the major shortcoming is the dearth of long-term placebo-controlled studies that would inform clinicians about persistence of efficacy or burden of adverse effects. Only two studies have been performed. In one, risperidone failed to have a significant effect in preventing relapse; however, methodological problems may have contributed to the result. A recent 26-week relapse prevention study of the olanzapine-fluoxetine combination showed a significant advantage for the combination. In that trial, after 6–8 weeks of acute treatment with olanzapine and fluoxetine and 12 weeks of stabilization, patients were randomly assigned to the combination or to fluoxetine plus placebo. A time-to-relapse analysis significantly favored the combination. Under the primary definition of relapse, 15.8% of the patients on the combination relapsed, compared with 31.8% of the patients on fluoxetine. Nevertheless, the paucity of long-term controlled studies for the atypical agents limits our understanding of the persistence of efficacy and of side effects that may increase or emerge with time. This leaves the clinician whose patient has responded to acute adjunctive treatment with little information for weighing the risks and benefits of continuing the adjunctive agent.
So that’s why I liked the Spielmans et al meta-analysis [the extra mile…]. It showed us that from the patients’ own ratings, they weren’t getting that much benefit – a very important piece of data. And for that matter, Spielmans et al documented the Adverse Events including the high incidence of Akasthisia [a Tardive Dyskinesia harbinger] with Abilify®. So I know why I’ve been so stuck on this business of Atypical Antipsychotic augmentation of the so-called treatment-resistant-depression. All of these Atypicals may have a low moderate effect on TRD symptoms, sure enough, but these drugs are time-bombs [particularly] if they are used long term [and they are used long-term!]. So if nobody’s really looked long term in the last 16 years, Why the hell not?
I hope the Yatham paper in Mol. Psych. inspires some copycats in the unipolar depression literature. The pharmaceutical companies are going to work very hard to market their drugs for a condition with ten times the prevalence of schizophrenia. It would be nice to have some actual evidence whether these augmentation effects are durable; or if they’re just accelerating what would otherwise happen on its own.
[I believe this is the paper Pat is referring to, 1BOM]
I have several people on my site who have developed tardive dyskinesia after being prescribed a “low” dose of an atypical antipsychotic for sleep or as “adjunct” to an antidepressant.
I’m thinking many of the people Altostrata has heard from were given the “Little Baby Dose Line.” That’s a marketing pitch aimed at calming the fears of patients who wonder if they should really be taking anti-psychotics for “incompletely remitted depression” — or even “treatment resistant depression” if it’s not totally disabling.
“Don’t worry. These are little baby doses we’re talking about — just a fraction of what we give people for schizophrenia!”
It works to calm nervous doctors too — pretty soon they are handing out “little baby doses” of neuroleptics as casually as they hand out Ambien or Ritalin. Only trouble is, it’s a bit of a scam. One reason: the competing metabolic pathways of these drugs can double or even triple your blood level of the antipsychotic when you take it in combination with certain antidepressants. A bit of expose on RxISK.org regarding this: http://rxisk.org/abilify-from-the-inside-out/
Very true, Johanna.
Hey, folks,
What is everyone seeing for the very first symptoms of TD that everyone is seeing? Are there any prodromal symptoms that occur before it sets in?
I know this is covered in a million different articles, but I’m much more interested in clinical observations. What I’ve seen that made me suspicious– and I saw it only a couple of times– was sort of a restless lower lip, like when people have been snorting lots of blow and/or a kind of simian lip puckering.
It came on very fast for my client, and he/she and the prescriber were both very dismissive of it.
Thanks,
Thanks.
Catalyzt,
I think it’s the stereotypy. There are multiple versions of abnormal mouth/tongue movements that may appear [“oro-buccal-lingual stereotypy“], but the thing that catches my attention is that whatever they are, they’re repeated.
Thank you, Mickey. I had not seen the term “stereotypy” before, although– strangely– “oro-buccal-lingual” was familiar.
The wiki on stereotypy in animals was very interesting. (Sorry to hijack the thread here– I know the topic is TD from augmentation with atypicals, but the environmental/cultural piece of this got my attention.)
Stereotypical behaviors are thought to be caused ultimately by artificial environments that do not allow animals to satisfy their normal behavioral needs. Rather than refer to the behavior as abnormal, it has been suggested that it be described as “behavior indicative of an abnormal environment.”[18] Stereotypies are correlated with altered behavioral response selection in the basal ganglia.[15] As stereotypies are frequently viewed as a sign of psychological distress in animals, there is also an animal welfare issue involved. (Yeah, no kidding!)
Stereotypical behavior can sometimes be reduced or eliminated by environmental enrichment, including larger and more stimulating enclosures, training, and introductions of stimuli (such as objects, sounds, or scents) to the animal’s environment. The enrichment must be varied to remain effective for any length of time. Housing social animals with other members of their species is also helpful. But once the behavior is established, it is sometimes impossible to eliminate due to alterations in the brain.
Johanna,
Thank you so much for the link you provided – that information has made a whole piece of what happened to us make so much sense! The site is truly an amazing service