I’m picking up the thread of a story in the middle, because I didn’t realize it was a story with installments until I was well into it. It started with December’s American Journal of Psychiatry that had two Publicly funded Clinical Trials that looked like Infommercials [Experommercials] to me, one being about augmenting antidepressants with Geodon® in the mythologic ‘treatment resistant depression.’ I was upset that it was publicly funded [creative funding I…, creative funding III & some other things…]. Looking at it, I noticed the lead author and the author of the accompanying editorial had done a meta-analysis of ‘augmentation’ in 2009. And so I looked at that meta-analysis which I thought was reasonable, but I disagreed with the conclusion [skepticism unchanged…]. That lead to a more recent [2013] metanalysis that was more extensive, one I agreed with [the extra mile…, a worksheet post…]. Then I ran across a pair of articles on a new Atypical Antipsychotic [brexpiprazole], virtual clones of each other. Brexpiprazole [Rexulti®] was recently approved for augmentation of ‘treatment resistant depression’ [extending the risk…, a postscript…]. That’s a story in my book, though I don’t yet have a title. This post picks up in the middle with those two brexpiprazole articles.
In extending the risk…, I couldn’t stop talking about these two articles being clones. The only difference is that one used two doses [1MG AND 3MG] and the other used only one [2MG]. Why didn’t they just do three doses in one study? We can answer one that right off the bat. The FDA requires two studies for approval, so that’s all there is to say about that. We’re so used to it that we don’t even register that this is a blatant example of a pharmaceutical company using our scientific literature for commercial purposes. They had different ghost-writers from the same firm, but they must’ve been in adjacent cubicles because big pieces of are actually cut and pasted. The Clinical Trials were submitted on the same day. I’m just going to mention a few of their differences. Here are the stats from clinicaltrials.gov:
| STUDY | CLINICAL TRIAL | START | STOP | N | SITES | ~N/SITE | ||||||
|
|
||||||||||||
| 1MG AND 3MG | NCT01360632 | JUN 2011 | SEP 2013 | 677 | 71-92 | 7-10 | ||||||
| 2MG | NCT01360645 | JUL 2011 | MAY 2013 | 379 | 57-59 | ~6 | ||||||
If you look at the SECONDARY OUTCOME VARIABLES, there is a big difference [which I’ll discuss later]. These studies are done by Contract Research Organizations [MGH?], and they have a huge number of SITES all over the world. They do this to speed things up, as any given SITE has to only recruit a small number of subjects [the possibility of introducing error is obviously increased by this ploy]. Here are the papers [full text available]. It’s worth your while to actually scan through them. I’ve named them [1MG AND 3MG and 2MG] for clarity:
1MG AND 3MGby Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, Nyilas M, Carson WH, Sanchez R, and Eriksson H.Journal of Clinical Psychiatry. 2015 76[9]:1232-1240.
2MGby Thase ME, Youakim JM, Skuban A, Hobart M, Augustine C, Zhang P, McQuade RD, Carson WH, Nyilas M, Sanchez R, and Eriksson H.Journal of Clinical Psychiatry. 2015 76[9]:1224-31.
Following the prospective treatment phase, patients were eligible for entry into the double-blind randomized treatment phase if they had inadequate prospective ADT response, defined as < 50% reduction in HDRS-17 total score between baseline and end of the prospective phase, with an HDRS-17 total score of ≥ 14 and a Clinical Global Impressions-Improvement scale (CGI-I) score of ≥ 3 at the end of the prospective phase. While this study was ongoing, additional analyses were performed on data from a completed phase 2 study of similar design… It was found that a small number of patients in that study had seemingly adequate improvement in Montgomery-Asberg Depression Rating Scale (MADRS) and CGI-I scores at various times during the prospective treatment period, but subsequent worse scores at time of randomization. These patients did not show a consistent lack of response and would have been considered adequate responders if evaluated at another time point during the prospective phase. A number of these patients showed significant improvement again during the randomized phase, even if continuing on ADT alone.
In order to exclude patients with seemingly variable response to ADT, this study’s protocol was amended in March 2012 during the enrollment phase and prior to database lock to specify that patients had to meet more refined inadequate response criteria throughout prospective treatment (HDRS-17 score ≥14, <50% reduction from baseline in HDRS-17 as well as <50% reduction in MADRS total score between start of prospective treatment and each scheduled visit, and CGI-I score ≥3 at each scheduled visit) to be eligible for randomization. The investigator was also blinded to the revised criteria. Both the protocol amendment and the resulting primary analysis were discussed and agreed with the relevant regulatory authorities (US Food and Drug Administration).
In order to exclude patients with seemingly variable response to ADT, this study’s protocol was amended to specify that patients had to meet more refined inadequate response criteria throughout prospective treatment (a HDRS-17 score ≥14; < 50% reduction from baseline in HDRS-17, as well as <50% reduction in MADRS total score between start of prospective treatment and each scheduled visit, and CGI-I score ≥3 at each scheduled visit) to be eligible for randomization and also to blind the investigator to the revised criteria.
"MADRS score (primary end point). In the efficacy population per final protocol, mean reduction from baseline to week 6 in MADRS total score for brexpiprazole 3 mg showed greater improvement (−8.29) compared with placebo (−6.33; least squares [LS] mean difference = −1.95; 95% CI, −3.39 to −0.51; P = .0079) (Figure 2). Mean change in MADRS total score for brexpiprazole 1 mg was −7.64 versus −6.33 for placebo (LS mean difference = −1.30; 95% CI, −2.73 to 0.13; P = .0737) (Figure 2).""Mean change in MADRS total score for the efficacy population also showed improvement for brexpiprazole 3 mg versus placebo (−1.52; 95% CI, −2.92 to −0.13; P = .0327) but did not reach the level of statistical significance required for multiple comparisons according to the prespecified statistical analysis. The mean improvement for brexpiprazole 1 mg versus placebo was less than that for 3 mg (−1.19; 95% CI, −2.58 to 0.20; P = .0925) (Supplementary eFigure 1)."
"MADRS score (primary end point). Mean reduction from baseline to week 6 in MADRS total score was greater for brexpiprazole compared with placebo (LS mean = −8.36 vs −5.15; LS mean difference = −3.21 [95% CI, −4.87 to −1.54], P = .0002; efficacy population per final protocol) with difference between treatment groups apparent from the first week onward (Figure 2).""Similar results were seen for brexpiprazole versus placebo in the efficacy population (LS mean = −8.27 vs −5.15; LS mean difference = −3.12 [95% CI, −4.70 to −1.54], P = .0001) (Supplementary eFigure 1)."
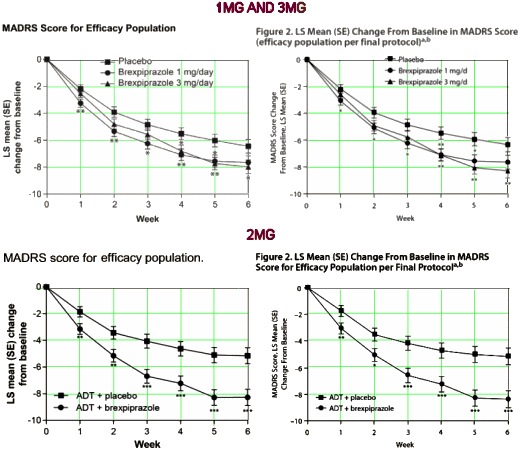
First, in 2MG they are on solid ground with either protocol. But that wasn’t true in 1MG AND 3MG. With the efficacy population, they report a P = .0327 for the 3mg dose in the narrative [above], but after correcting for false-discovery rate with multiple variables with the protocol specified Hochberg correction [too many SECONDARY OUTCOMES], both 1mg and 3mg are insignificant. With the efficacy population per final protocol, the 3mg dose achieves significance. If you look at those upper graphs long enough, you can see the difference – slight, subtle, just enough to pull the numbers into significance [don’t squint]. And remember, they need two positive studies for FDA approval.
Thase is outdoing himself. Dr. Carlat called him the man who put Effexor on the map, which put Thase on my Wanted for Questioning list. I recently learned he was involved in Vortioxetine (Brintellix), Lundbeck/Takeda’s #NextBigThing last year, which turned out to be a side-effects sampler that absolutely no one stays on for more than 4 weeks, except a certain kind of forum member who joins a site, posts a positive and insightful review the same day, posts nothing else, and is not heard from again. Brintellix will eventually find an off-label indication for patients allergic to syrup of ipecac. Brutal headaches, relentless itching, and not working round out its feature set. It is not terribly surprising that he’s involved in this folly. When studies showing that antidepressants don’t contribute much beyond what placebo offers started popping up, he took the line that when millions of people get marginal help, it adds up to a lot of help.