
ReutersBy Bill BerkrotNovember 22, 2011Investors in Gilead Sciences may believe the company is paying too much to buy Pharmasset Inc VRUS.O at $11 billion. Emory University researcher Raymond Schinazi, who founded Pharmasset, knows by just how much. "They could have had the company for $300 million or less in 2004. Somebody made a huge mistake," Schinazi said in a telephone interview a day after the big deal was announced.
Schinazi, 61, is the largest individual shareholder of Pharmasset and saw the value of his own 4 percent stake leap to more than $440 million. The biotech is developing a hepatitis C treatment considered so promising that Gilead agreed to an 89 percent premium over Pharmasset’s already soaring stock price. Schinazi said he was surprised that Gilead, the world’s largest maker of HIV drugs and a company well-versed in antiviral treatments, did not recognize what Pharmasset was offering when it solicited offers back in 2004. "Now they paid the premium. Of course, now the risk has been reduced significantly," he said of the drug that has demonstrated impressive results in clinical trials…
Something of a one-man bridge between the worlds of academic and commercial science, Schinazi is director of the Emory University Center for AIDS Research and a star in the world of HIV research and treatment. He has also founded five companies developing treatments for viral diseases such as AIDS, hepatitis and herpes…
He has not been involved in running Pharmasset since 2006 and had no part in the Gilead deal announced on Monday. But he was chairman of the board and led its chemistry group when the molecule that led to the hepatitis treatment was discovered…
Pharmalot: WSJby Ed Silverman06/29/2015A pair of public health advocacy organizations has filed a lawsuit against the FDA, claiming the agency failed to release clinical trial data for Gilead Sciences hepatitis C treatments on a timely basis. And the move is only the latest installment in an ongoing drama in which researchers and patient advocates have tussled with drug makers and regulators over access to such information.
Here’s what happened: Late last year, Treatment Action Group and the Global Health Justice Partnership asked Gilead for patient-level trial data for the Sovaldi and Harvoni drugs. They sought the data because the drugs are widely prescribed, thanks to very high cure rates, and because the FDA approved the drugs as part of a regulatory process known as a breakthrough designation, which accelerated review…
In their lawsuit, the groups maintain doctors “lack the benefit of any independent assessment of the data.” And given the high cost of the drugs, the groups argue in their lawsuit that it is “crucial that policymakers be able to evaluate the cost-effectiveness… based on the underlying clinical data…” Sovaldi and Harvoni cost $84,000 and $94,500, respectively, for 12-week regimens, before discounts. But Gilead never replied to their requests last November for trial data, according to the lawsuit.
So last December, the groups turned to the FDA and submitted a Freedom of Information request for the data, since the drug maker had submitted the information to the agency as part of the drug approval process. However, the groups say the FDA denied their request for “expedited processing” and maintained it would take from 18 to 24 months to fork over the data, according to the lawsuit…
"When I tried to look at more recent drugs, things were less smooth. For one of the last requests I submitted, I got a call from the FDA and the FDA lady tried to talk me out of making the request in the first place. When I refused and asked her how long it would take, she said about two years [that was three years ago] and I never heard from them again."
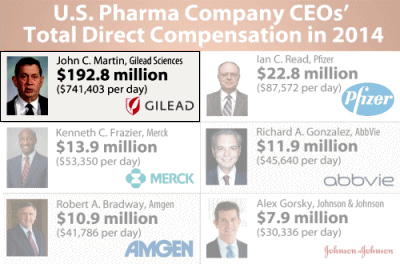
The irony here is obvious. We give Gilead an FDA break for the R&D work they didn’t do, fast track them to patent protection, so they can charge an outrageous fee to patients with Hepatitis C [most of whom can’t afford it so governments end up paying] so they can pay their head honcho $192+ M as a yearly salary, but we can’t expedite the NDA report from the FDA to allow some attempt at vetting the drug. I reckon Gilead has cut its business teeth on squeezing people with AIDS, and really knows how to play the system…
Gee, doesn’t seem to refute my point there is no profit margin, at the end of the day, in health care that is responsible and appropriate, eh?
As an addendum to the above comment, I read this Letter to the Editor in the June 2015 issue of Clinical Psychiatry News, from Kathleen Hickey, MD, about shame as a motivator in medicine must end (and per my somewhat exhaustive efforts to find a link, none at the site as of now). In it, she notes how a hospital she worked for had a list of all providers along with the number of relative value units and dollar amounts billed each month, and they were categorized by color depending on where they fell on a national standard. Yes, it was provided to all staff on a monthly basis, and the intent was overtly to shame to thus motivate providers to see more patients.
What exactly is the point of using shame for nefarious ends? It is an antisocial agenda, in my opinion, and thus once again shows why we as physicians need to not only refute this BS agenda to use profit solely as the driving force to “health care decisions” when in fact it is about bottom lines, and usually the ones fattening the wallets of those who don’t do squat as a provider; instead, we need to call it out and reverse the shame agenda and make the communities these charlatans operate in return the shame these liars and cheats who are just profit driven slime are about!
Oh yeah, my bad, shame and integrity, where are they these days, buried in some unmarked graves next to common sense and communal decency.
The obscene fact that Hillary Clinton is still a viable candidate for President of the United States shows we have an entrenched electorate who has no clue to not only right versus wrongs, but, an obscene tolerance for lying and remorselessness. Yep, the same standards of most hospital administrators.
So, to wrap up this comment here, what does Big Pharma learn from this loser leading Gilead? “How do we replicate this money maker?!”
And the whores and cowards in medicine just march goose step in adherence to the profit driven agendas, on and on…
And to recoup their expenditure, I’d wager that it will be recommended for anyone diagnosed with Hep C, even though it’s probably not necessary for people who have no evidence of liver damage. The differences between risk and risk factors should be spelled out as a critical component of informed consent.
but then remember that big Pharma, as all big business effectively print money in the currency called shares.
http://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/americans-want-to-pop-made-in-india-sovaldi-drug-for-1-per-cent-of-the-cost/articleshow/47521457.cms
OK, who here wants to kickstart a medical tourism business transporting patients to Bangalore (staying in a four star hotel) for 1% of the treatment cost here? This is certainly cheaper than paying for a course of treatment in the US even factoring transportation and lodging. I can’t imagine PBMs wouldn’t greenlight this given the savings. Win-win.