I’ve left out a lot – Wayne McFadden’s affair with a Parexel writer, more buried trials [Trial 0031, Trial 0041, etc.], the new XR campaign to stay in the game with depression, and a bunch of email threads showing
AstraZeneca‘s corrupt corporate climate. In one of the comments,
PharmaGossip mentions
complicity theory:
“Complicity works like this. All those with a vested interest in an enterprise get sucked into the rhetoric associated with it, and they soon `believe’ in everything that is going on within that enterprise. If personal financial gain is involved, corruption may also occur.” – Paul Dieppe
I guess that explains it as well as any other, and is more palatable than the occasional demonic theories that have occurred to me along the way. I look forward to reading the book somebody is going to write someday, fleshing out all the details of the
Seroquel story, but for the moment, I think I’ve made my point. I guess I sort of knew something about Pharmaceutical Companies before I started looking into all of this. What I knew nothing about were CROs [
Clinical Research Organization AKA
Contract Research Organization] [they’ve even got a club,
ACRO]. It’s the ultimate outsourcing solution. The CRO arranges and manages the clinical trials including writing the paper, finding authors to sign on, submitting the articles, etc. in whatever combination of services you want to pay for. They, in turn, interact with multiple Centers that recruit the subjects and do the studies – usually multiple centers in a given clinical trial. This is something of a new, multibillion dollar industry. Here’s the pitch from
Parexel, the CRO used by
AstraZeneca:
The pressure to accelerate time-to-market for innovative pharmaceutical products has never been more intense. Every biopharmaceutical company feels the need to drive promising therapies through development and gain market approval as quickly as possible to maximize the value of its assets. At the same time, current market and economic realities are compelling the industry to reduce fixed costs, increase efficiency, and concentrate limited resources on core competencies. The challenge of accelerating pharmaceutical product development while controlling costs creates a difficult balancing act for industry executives. Leveraging strategic pharmaceutical research development partnerships allows bio/pharmaceutical companies to greatly increase operational efficiency by focusing their resources on the highest priorities – such as discovery research and product marketing – while utilizing the specialized expertise and infrastructure of their partners for other services.
As you can see, they’ve got a business lingo and they are postured to stay in the background, though in practice they are very much in charge of the "time-to-market" process. The final output is in the stream of articles we’ve been looking at in this and the earlier series, which I think of as the:
least·mean·square·intention·to·treat·last·observation·carried·forward·placebo·versus·drug·rating·scale
graphs [CRO-Charts for short]. These CRO-Charts are pretty monotonous.
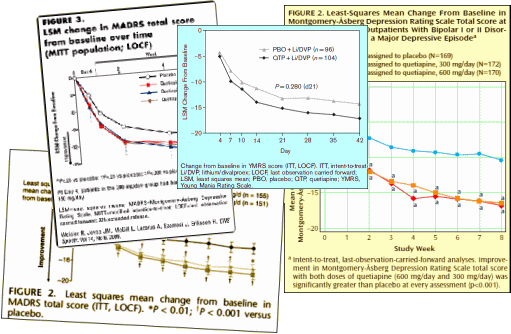
Most of them have a dramatic placebo effect. Speaking of drama, there are very high drop-out rates in these studies [40-80%]. Some of the drooping placebo plot is probably due to people with worsening symptoms dropping out, selecting for the naturally improving subjects – but one is always left with the nagging suspicion that there are people in the studies who weren’t very "sick" in the first place. Recall that many of these subjects are recruited by magazine and newspaper ads. Then there are the treated subjects with a statistically significant response curve [which is not very dramatic]. One interesting thing is that in the studies with multiple doses, there is often no difference between the different doses, which I find odd. So the question comes down to, "Is a statistically significant response compared to an improving placebo group in a 6 week study where the majority of subjects drop out before the study is over a way to determine if a drug is
INDICATED in the treatment of a disorder?" One can elaborate the question further, "if the study was managed by a CRO hired by the maker of the drug, the lead scientific authors have never seen the primary data, and, in all likelihood, those authors did not write the paper [
selling seroquel VII: indication sprawl…]?" And then there’s another question. Many of these approved indications are class specific – something one would expect from the whole group of medications. If
Seroquel is an effective add-on drug for patients with Major Depressive Disorders [which I personally question], would that differentiate it from the other Atypical Antipsychotics? I doubt it. In each of the cases in the last post, when another drug does a "
me too" trial, their drug is effective too [or at least produces equally favorable
CRO-Charts]. When
AstraZeneca says "
Seroquel XR is now approved for …" as if that’s something specific about
Seroquel XR, it seems meaningless. So why is the F.D.A. allowing their approvals for secondary indications be used in competitive marketing schemes between Pharmaceutical Companies?
And about AstraZeneca, it’s a company that exists to make money. There’s no complaint about them wanting to maximize the profit from their product. The complaint is that they are willing to do that at the expense of the patients who take their drug. They actively hid the fact that their drug regularly causes weight gain and sometimes Diabetes for seven years. They stuck with "placebo-like" EPS for years beyond the time when it was obviously not true. They buried studies that either showed minimal [or no] efficacy or an unflattering incidence of side effects. They published any number of CRO-Chart articles under the names of respected researchers that were largely their own productions. They actively promoted their drug "off-label" and pushed "class specific" indications as if they owned them. They capitalized on the lower incidence of Tardive Dyskinesia to expose more patients to that risk than would ever occur with the first generation drugs because of their wide range of supposed indications. And they gambled successfully that the damage they did to people [Diabetes, Weight Gain, Tardive Dyskinesia] would cost them less than the profit they made by keeping the dangers of their drug secret. There’s no excuse for that.

Obviously, Psychiatry needs to clean up its act [again]. Right now, there’s a lot to focus on – ghostwriting, jury-rigged speaker’s bureaus, product-slanted C.M.E. presentations, undisclosed conflicts of interest, Pharmaceutical Industry driven academic research, a scientifically unsupportable Diagnostic Manual, etc. What the
Seroquel story adds is deceitful stealth marketing and
AstraZeneca‘s pushing the current Clinical Trial paradigm into the realm of absurdity. Once a Clinical Trial has been registered and completed, it should not be possible to bury it. Raw Data needs to be available not just to the CRO, but also to authors, and to the rest of us. The opaque
CRO-Charts are not sufficient – there’s been enough abuse of true
evidence-based medicine to justify insisting that the data is available to people who want assess the validity of what’s published. And there’s a need for
research into how to actually determine
clinical relevance in evaluating the efficacy and safety of the medications we offer our patients. Statistical significance in a
CRO-Chart is simply not enough. That’s my take-home from reading the published versions of these
Seroquel Clinical Trials. It has become a "time-to-market" racket…

 Obviously, Psychiatry needs to clean up its act [again]. Right now, there’s a lot to focus on – ghostwriting, jury-rigged speaker’s bureaus, product-slanted C.M.E. presentations, undisclosed conflicts of interest, Pharmaceutical Industry driven academic research, a scientifically unsupportable Diagnostic Manual, etc. What the Seroquel story adds is deceitful stealth marketing and AstraZeneca‘s pushing the current Clinical Trial paradigm into the realm of absurdity. Once a Clinical Trial has been registered and completed, it should not be possible to bury it. Raw Data needs to be available not just to the CRO, but also to authors, and to the rest of us. The opaque CRO-Charts are not sufficient – there’s been enough abuse of true evidence-based medicine to justify insisting that the data is available to people who want assess the validity of what’s published. And there’s a need for research into how to actually determine clinical relevance in evaluating the efficacy and safety of the medications we offer our patients. Statistical significance in a CRO-Chart is simply not enough. That’s my take-home from reading the published versions of these Seroquel Clinical Trials. It has become a "time-to-market" racket…
Obviously, Psychiatry needs to clean up its act [again]. Right now, there’s a lot to focus on – ghostwriting, jury-rigged speaker’s bureaus, product-slanted C.M.E. presentations, undisclosed conflicts of interest, Pharmaceutical Industry driven academic research, a scientifically unsupportable Diagnostic Manual, etc. What the Seroquel story adds is deceitful stealth marketing and AstraZeneca‘s pushing the current Clinical Trial paradigm into the realm of absurdity. Once a Clinical Trial has been registered and completed, it should not be possible to bury it. Raw Data needs to be available not just to the CRO, but also to authors, and to the rest of us. The opaque CRO-Charts are not sufficient – there’s been enough abuse of true evidence-based medicine to justify insisting that the data is available to people who want assess the validity of what’s published. And there’s a need for research into how to actually determine clinical relevance in evaluating the efficacy and safety of the medications we offer our patients. Statistical significance in a CRO-Chart is simply not enough. That’s my take-home from reading the published versions of these Seroquel Clinical Trials. It has become a "time-to-market" racket…
You ARE writing the book! Time to find an agent and publisher.
This paper is well worth a read!
http://pharmagossip.blogspot.com/2011/03/those-who-have-gold-make-evidence-how.html