Among the stories that have emerged over the last twenty years in this age of psychopharmacology, few are as strange as the story of John Rush who was in the Department of Psychiatry at the University of Texas Southwestern for thirty years. Early on, he had studied Cognitive Behavior Therapy with Aaron Beck but not long after arriving at UT, he became a researcher in Depression focusing on psychopharmacology. He was successful in getting NIMH grants as well as industry funding and held various research positions in the Department. Somewhere around 1995, UT and TDMHMR, the State Mental Health Agency, decided to team up in what would become the
TMAP program. In the recent Janssen Trial,
Dr. Shon Medical Director of TDMHMR described it this way:
Q. Okay. So this — this notion of the — the cooperation and working together — between the research universities and the public sector?
A. That’s correct.
Q. What did that lead to?
A. And that had been going on, but this was my first kind of injection into dealing with that part of the department. So I went up to the Department of Psychiatry in Dallas, UT Southwestern, and met with Ken Altschuler and John Rush, who were the two people who – Ken Altschuler was the chair of the department at the time and John Rush a researcher there. And we met one afternoon and talked about what kind of projects we might focus on, because one cycle of the projects had come to an end and we were ready to start another cycle. So we kind of narrowed it down to a couple of areas. One was psychosocial interventions and the other was medication interventions. And it was out of that meeting that the decision was made to focus on improving the quality of medication prescribing. And that was one of the things that we had talked about because one of the things that I had seen as medical director in reviewing prescribing in our state, and it was really not different in California, was that prescribing could be fairly erratic. It was not consistent at all. One of the examples I would give as I gave talks is that if you had six people who had the same symptoms, everything was the same, perhaps they were clones and had the same psychiatric disorder, and they walked into six psychiatrists’ offices all lined up, chances were fairly high that they would walk out with six different medication programs.
Q. So this — this issue of the erratic or inconsistent prescribing of medications for mental illness, that’s — that’s the — that’s one of the topics that you discussed at this meeting at UT Southwestern?
A. Yes.
Q. And that was in what year?
A. At that meeting in ’95, ’96…
However it came to be, Dr. Rush became TMAP Director and was in charge of starting and running the program. This from the deposition of
Nancy Burch-Smith, a Janssen employee in charge of disbursing grants:
…When requested, we at times would fund Dr. Shon to share his – his novel concept of TMAP with other states.
Q. And you say his novel concept?
A. I see him as the conductor or creator, main developer of TMAP.
…
Q. What do you recall your initial support being?
A. My recollection was that Dr. Rush said that he was approaching every company I believe for the amount of $75,000.
Q. Well, you knew from Janssen’s perspective that what Janssen was hoping to get from the support of TMAP was favorable Risperdal positioning within the TMAP algorithm; isn’t that right?
A. I think ultimately we supported TMAP because we totally believed in what TMAP stood for. Where — when we agreed to the $75,000, we had no idea where Risperdal or any of the atypicals would end up on the algorithm. But, again, it would only be natural – I work for a for-profit company, that it would be natural that if we had favorable positioning, it would ultimately help sales. But that was not our primary objective in looking at TMAP…
But there’s little question that Dr. Rush was involved with Janssen’s marketing efforts in the earliest days of TMAP’s implementation. This is from EKS, the group that developed the TriUniversity Schizophrenia guidelines that were incorporated into TMAP, in a
1996 note to Janssen:
"EKS is now ready to move forward in a strategic partnership with Janssen." The strategy will allow Janssen to "Influence state governments and providers… Build brand loyalty and commitment with large groups of key providers around the country." EKS also promised "rapid implementation," with particular attention to having an impact on Texas decision making. "It is our intent to work with the State of Texas immediately in implementing this product in a select number of CMHC’s with the assistance of A. John Rush, MD"… In its summary of the document, EKS wrote: "Your investment in the development of state of the art practice guidelines for schizophrenia is already beginning to pay off in terms of positive exposure in the Texas implementation project."
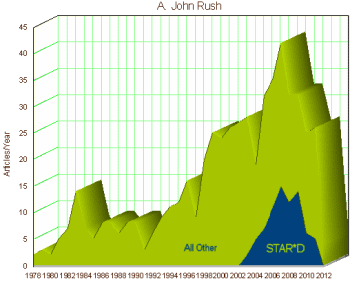
So Dr. Rush directed the TMAP program, a Depression researcher more involved with the depression algorithm with UT colleague, Dr. Madhukar Trivedi, than the Schizophrenia Algorithm itself [the focus of the Janssen Trial]. Presumably bolstered by his work with the TMAP Algorithms, in 1999 he received an NIMH grant to do the largest clinical trial of a sequenced treatment algorithm for Depression ever done, known as
STAR*D, with Dr. Trivedi as his assistant P.I. [
a thirty-five million dollar misunderstanding…]. As you can see in the opening graph, Dr. Rush’s publication rate soared during the period of TMAP and STAR*D [including his developing a rating scale (QIDS) specifically for this study].
But then things took a downward turn. Allen Jones filed his lawsuit alleging that Janssen had misbehaved badly in its involvement with TMAP and the State of Texas joined the suit. TMAP itself began to dissemble. The papers generated by STAR*D continued to flow, but the definitive results were not forthcoming. When they were finally published, they were, candidly, a garbled mess. Rush’s QIDS was used rather than the expected primary outcome variables, HAM-D. And while derivative STAR*D papers keep coming, the core findings remain unknown to this day. Rush’s fortune took a further downturn when he was listed by Senator Grassley as one of the psychiatric Key Opinion Leaders with unreported industry income. That year, he left UT and moved to the Duke medical graduate program in Singapore. A later study of his, CO-MED, using multiple antidepressants was a bust – no improvement. TMAP is now a largely forgotten program – except in litigation circles. And the results of STAR*D are still essentially unknown – a $35 M study that produced its own library of articles, but made no contribution to science that I’m aware of.
Now, Dr. Ed Pigott, a psychologist who has spent several years studying the deficiencies in STAR*D has filed a whistleblower suit accusing Dr. Rush of having had an inappropriate financial relationship with Forest Laboratories, the maker of Celexa, the first order antidepressant used in STAR*D [
let the discovery begin…]. Having spent some time looking at STAR*D myself, I can only agree with each of Dr. Pigott’s points about the study itself [
the suit]. I found it baffling. And then there’s this – the COI declarations from the definitive report of the results – a rogues gallery of KOLs [including the boss of the DSM-5 Task Force]:
Dr. Rush has served as an advisor, consultant, or speaker for or received research support from Advanced Neuromodulation Systems, Inc.; Best Practice Project Management, Inc.; Bristol-Myers Squibb Company; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals, Inc.; Gerson Lehman Group; GlaxoSmithKline; Healthcare Technology Systems, Inc.; Jazz Pharmaceuticals; Merck & Co., Inc.; the National Institute of Mental Health; Neuronetics; Ono Pharmaceutical; Organon USA Inc.; Personality Disorder Research Corp.; Pfizer Inc.; the Robert Wood Johnson Foundation; the Stanley Medical Research Institute; the Urban Institute; and Wyeth-Ayerst Laboratories Inc. He has equity holdings in Pfizer Inc and receives royalty/patent income from Guilford Publications and Healthcare Technology Systems, Inc.
Dr. Trivedi has served as an advisor, consultant, or speaker for or received research support from Abbott Laboratories, Inc.; Akzo (Organon Pharmaceuticals Inc.); Bayer; Bristol-Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Johnson & Johnson PRD; Meade Johnson; the National Institute of Mental Health; the National Alliance for Research in Schizophrenia and Depression; Novartis; Parke-Davis Pharmaceuticals, Inc.; Pfizer Inc; Pharmacia & Upjohn; Predix Pharmaceuticals; Sepracor; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories.
Dr. Wisniewski has received research support from the National Institute of Mental Health and served as an advisor/consultant for Cyberonics, Inc.
Dr. Nierenberg has served as an advisor, consultant, or speaker for or received research support from Bristol-Myers Squibb Company; Cederroth; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals Inc.; Genaissance; GlaxoSmithKline; Innapharma; Janssen Pharmaceutica; Lichtwer Pharma; the National Institute of Mental Health; the National Alliance for Research in Schizophrenia and Depression; Neuronetics; Organon, Inc.; Pfizer Inc; Sepracor; Shire; Stanley Foundation; and Wyeth-Ayerst Laboratories.
Dr. Stewart has served as an advisor, consultant, or speaker for or received research support from Eli Lilly & Company; GlaxoSmithKline; Organon USA Inc.; Shire; and Somerset.
Dr. Warden has received research support from the National Institute of Mental Health and has equity holdings in Bristol-Myers Squibb Company and Pfizer, Inc.
Dr. Thase has served as an advisor, consultant, or speaker for AstraZeneca; Bristol-Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Laboratories, Inc.; GlaxoSmithKline; Janssen Pharmaceutica; Eli Lilly & Company; Novartis; Organon, Inc.; Pfizer Pharmaceutical; Sanofi Aventis; Sepracor, Inc.; Shire US Inc.; and Wyeth Pharmaceuticals.
Dr. Lavori has served as an advisor, consultant, or speaker for or received research support from Bristol-Myers Squibb Company; Celera Diagnostics Inc; Cyberonics, Inc.; the Department of Veterans Affairs; Forest Pharmaceuticals, Inc.; Glaxo-SmithKline; Leaf Cabrezer Hyman and Bernstein; the National Institutes of Health; and Neuronetics, Inc.
Dr. McGrath has served as an advisor, consultant, or speaker for or received research support from Eli Lilly & Company; GlaxoSmithKline; Lipha Pharmaceuticals; the National Institute of Mental Health; the National Institute on Alcohol Abuse and Alcoholism; New York State Department of Mental Hygiene; Organon, Inc.; Research Foundation for Mental Hygiene (New York State); and Somerset Pharmaceuticals.
Dr. Rosenbaum has served as an advisor, consultant, or speaker for or received research support from Astra-Zeneca; Boehringer-Ingelheim; Bristol-Myers Squibb Company; Cephalon; Compellis; Cyberonics; EPIX; Forest; GlaxoSmithKline; Janssen; Lilly; MedAvante; Neuronetics; Novartis; Orexigen; Organon; Pfizer, Inc; Roche Diagnostics; Sanofi; Schwartz; Somaxon; Somerset; Sepracor; Shire; Supernus; and Wyeth. He has equity holdings in Compellis, Medavante, and Somaxon.
Dr. Sackeim has served as an advisor, consultant, or speaker for or received research support from Cyberonics, Inc.; Eli Lilly & Company; Magstim Ltd.; MECTA Corporation; Neurocrine Biosciences Inc.; Neuronetics Inc.; NeuroPace Inc.; and Pfizer Inc.
Dr. Kupfer has served as an advisor, consultant, or speaker for or received research support from Amersham; the Commonwealth of Pennsylvania; Corcept Corporated; Eli Lilly & Company; F. Hoffmann-La Roche Ltd.; Forest Pharmaceuticals; Lundbeck; the National Institute of Mental Health; Novartis; Pfizer, Inc; Servier Amerique; and Solvay/Wyeth. He has equity holdings in Body Media and Med Avante and receives royalty income from Oxford University Press.
Dr. Fava has served as an advisor, consultant, or speaker for or received research support from Abbott Laboratories; Alkermes; Aspect Medical Systems; Astra-Zeneca; Bayer AG; Biovail Pharmaceuticals, Inc.; BrainCells, Inc.; Bristol-Myers Squibb Company; Cephalon; Compellis; Cypress Pharmaceuticals; Dov Pharmaceuticals; Eli Lilly & Company; EPIX Pharmaceuticals; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals Inc.; GlaxoSmithKline; Grunenthal GmBH; J & J Pharmaceuticals; Janssen Pharmaceutica; Jazz Pharmaceuticals; Knoll Pharmaceutical Company; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck; MedAvante, Inc.; Novartis; Nutrition 21; Organon Inc.; PamLab, LLC; Pfizer, Inc; PharmaStar; Pharmavite; Roche; Sanofi/Synthelabo; Sepracor; Solvay Pharmaceuticals, Inc.; Somerset Pharmaceuticals; and Wyeth-Ayerst Laboratories. He has equity holdings in Compellis and MedAvante.
Dr. Niederehe, Dr. Lebowitz, and Mr. Luther report no competing interests.
Symptom remission is the desired goal of treatment for depression, given its implications for better daily functioning and better longer-term prognosis. Since no treatment is a panacea, several sequential treatment steps are often needed to obtain remission with a tolerated treatment. If a trial does not result in remission, it is an unsuccessful trial, whether due to lack of efficacy or intolerable side effects, as long as the treatment is vigorously dosed to tolerance and provided for a sufficient duration to achieve remission. The number of treatment steps needed to achieve an adequate benefit is typically used to gauge the degree of treatment resistance, usually with a focus on acute outcomes without reference to longer-term outcomes.
And concludes:
After two treatment steps, it appears that over 50% of patients will achieve remission if they stay in treatment (i.e., 36.8% step 1 plus 30.6% of the remaining 63.2% of patients). Thereafter, the chances of subsequent remission are much lower. The theoretical cumulative remission rate after four acute treatment steps was 67%.
As I scroll through Dr. Rush’s 109 page CV – over 500 articles, more than 600 meeting presentations, numerous honors, awards, grants, book chapters, speeches, etc. – I’m wondering how it all came down to directing the corrupt TMAP program and submitting this indefensible conclusion to his STAR*D study – a study now sitting under the cloud of corruption. I also wonder why the NIMH who funded STAR*D hasn’t insisted that Dr. Rush et al publish the actual outcome data from the study or even evaluated the results themselves. I’m embarrassed that a watchdog has had to come from outside psychiatry, rather than from within our own ranks [though I’m glad it finally came from somewhere].
Dr. Rush and I are within a year in age, and our careers roughly paralleled up until the time when the DSM-III was published and psychiatry changed so dramatically. As much as I’d like to demonize him for falling in with the whole PHARMA-driven deterioration of academic medicine and his participation in shady enterprises like TMAP and STAR*D, I still find myself wondering about his journey through the last twenty-five years in the main stream of modern psychiatry. What the hell happened? He followed every lead along the road of depression research – Cognitive Behavior Therapy, most of the Drugs, the DST and other neurochemistry, the VNS system, ECT, etc, etc. He worked and published with most of the luminaries in the world of academic psychiatry over his career. But what was the point of amassing all of those publications, presentations, etc? How did he end up administering a Public Health program like TMAP that was little more than a tawdry PHARMA scam? Or squeezing that stream of extra articles out of the STAR*D study without ever addressing what the study was about in the first place? Or abruptly heading for Singapore at the end of a thirty year career in Texas when he should’ve been getting a watch at a retirement party? Why did he sign on to the iSPOT study with the likes of Nemeroff and Schlatzberg, chasing the next depression research dead end?
Sometimes when people say, "I don’t understand," it’s code for "I don’t approve." Well I obviously don’t approve, but also "I don’t understand" – for real…

 So Dr. Rush directed the TMAP program, a Depression researcher more involved with the depression algorithm with UT colleague, Dr. Madhukar Trivedi, than the Schizophrenia Algorithm itself [the focus of the Janssen Trial]. Presumably bolstered by his work with the TMAP Algorithms, in 1999 he received an NIMH grant to do the largest clinical trial of a sequenced treatment algorithm for Depression ever done, known as STAR*D, with Dr. Trivedi as his assistant P.I. [a thirty-five million dollar misunderstanding…]. As you can see in the opening graph, Dr. Rush’s publication rate soared during the period of TMAP and STAR*D [including his developing a rating scale (QIDS) specifically for this study].
So Dr. Rush directed the TMAP program, a Depression researcher more involved with the depression algorithm with UT colleague, Dr. Madhukar Trivedi, than the Schizophrenia Algorithm itself [the focus of the Janssen Trial]. Presumably bolstered by his work with the TMAP Algorithms, in 1999 he received an NIMH grant to do the largest clinical trial of a sequenced treatment algorithm for Depression ever done, known as STAR*D, with Dr. Trivedi as his assistant P.I. [a thirty-five million dollar misunderstanding…]. As you can see in the opening graph, Dr. Rush’s publication rate soared during the period of TMAP and STAR*D [including his developing a rating scale (QIDS) specifically for this study].
I sincerely ask this question of you, Mickey, as you are in the peer group of these colleagues who have been major players to the dismantling of psychiatry these past 15 years: those psychiatrists over 65 years old now, was there some sort of vetting process in residency programs back in the 1960s-early 80s that selected as many narcissistic/patriarchal attitudes and behaviors that alleged they would make better psychiatrists? That is not a sweeping generalization comment, but, after having been nearly ruined professionally at least three separate times in my career by colleagues older than 65, what the hell is going on with older colleagues that makes a sizeable number of them basically a danger to the patients and caring colleagues who are asking and expecting accountability and responsibility? Is losing credentials and treatment interventions making people become near sociopathic acceptable to the profession in large?
Not with me obviously!!!
Perhaps Dr. Rush found the road to riches. Or maybe he enjoyed being a paper mill.
I hated STAR*D from the initial study design. It proposed to switch thousands of people from drug to drug with no controls for withdrawal symptoms, thus replicating a central error of every antidepressant discontinuation since the beginning of time and guaranteeing invalid results.
(I was also surprised by the starring role of Celexa.)
However, the damage has been done by the publicity surrounding the release of its findings. Many clinicians believed it proved something. It made an imprint on the popular imagination. STAR*D was even mentioned as the gold standard by the usually astute Louis Menand in the New Yorker a few years back.
It’s a gold standard, all right — of everything that’s wrong with contemporary psychiatry. And why doesn’t NIMH release the underlying data? Doctors should put a public petition together and give it to Insel at some big press opportunity.
Thank you for covering this scandal. The darkest days of psychiatry may, one can hope, be coming to a close though with many killed by psych. drugs, including my son. What I wonder is where are the criminal prosecutions of those in the pharmaceutical industry and academic psychiatry who were willing to sell their souls for cash? And when will psychiatry push for what is necessary – alternatives to dangerous, lethal psych drugs …back to talking therapies and multidisciplinary non-drug alternatives( such as are described in Robert Whitaker’s excellent book “Anatomy of an Epidemic”.?) As long as those in the profession continue their complacency, the harmful status quo will go on. Where are your voices, or are you too busy with practices where patients are seen every 15 minutes and insurances charged?
With any luck, the Foundation for Excellence in Mental Health Care and other groups which have seen the light will gain more and more attention and the times will be changing soon.
I’m so happy to see continued coverage on the corruption of the scientific literature in psychiatry. Thanks Doc.