Well chasing down the leads from the last post was disappointing. Brain Resource [the sponsor of iSPOT] does indeed have a data sharing plan, but it’s not sharing of a particular study like iSPOT. It’s a related but separate organization called BRAINnet. BRAINnet is a large database of neuroscientific data on both normal and a variety of patients diagnosed with various mental illnesses including neuroimaging, EEG, biochemical, and neuropsych data [from Brain Resource]. It’s free, well sort of. You have to apply to have access, actually you have to be recommended by an existing member. It’s pretty complicated, but for our purposes, it’a a data sharing proposition [personalized medicine: BRAINnet…, personalized medicine: the Brain Resources company I…, and personalized medicine: the Brain Resources company II…].
by Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, and Fava M.American Journal of Psychiatry. 2006 Nov;163[11]:1905-17.
OBJECTIVE: This report describes the participants and compares the acute and longer-term treatment outcomes associated with each of four successive steps in the Sequenced Treatment Alternatives to Relieve Depression [STAR*D] trial.METHOD: A broadly representative adult outpatient sample with nonpsychotic major depressive disorder received one [N=3,671] to four [N=123] successive acute treatment steps. Those not achieving remission with or unable to tolerate a treatment step were encouraged to move to the next step. Those with an acceptable benefit, preferably symptom remission, from any particular step could enter a 12-month naturalistic follow-up phase. A score of <or=5 on the Quick Inventory of Depressive Symptomatology-Self-Report [QIDS-SR[16]] [equivalent to <or=7 on the 17-item Hamilton Rating Scale for Depression [HRSD[17]]] defined remission; a QIDS-SR[16] total score of >or=11 [HRSD[17]>or=14] defined relapse.RESULTS: The QIDS-SR[16] remission rates were 36.8%, 30.6%, 13.7%, and 13.0% for the first, second, third, and fourth acute treatment steps, respectively. The overall cumulative remission rate was 67%. Overall, those who required more treatment steps had higher relapse rates during the naturalistic follow-up phase. In addition, lower relapse rates were found among participants who were in remission at follow-up entry than for those who were not after the first three treatment steps.CONCLUSIONS: When more treatment steps are required, lower acute remission rates [especially in the third and fourth treatment steps] and higher relapse rates during the follow-up phase are to be expected. Studies to identify the best multistep treatment sequences for individual patients and the development of more broadly effective treatments are needed.
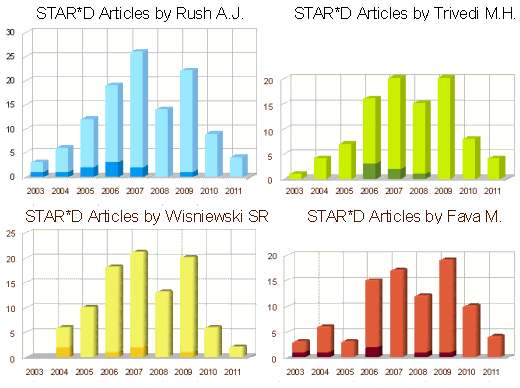
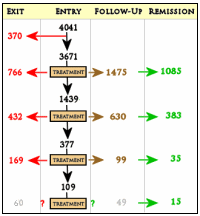 In spite of milking the database, the worth certainly wasn’t in the reports on the primary outcome. The statement in red above leaves out a small detail, the patients disappeared at an impressive rate. I never could get the exact figures, but the graphic on the right is close. It was impossible to conclude how many actually remained at the end of each level. But the actual numbers didn’t come close to the numbers reported in the abstract. In addition, they changed rating scales in mid-stream. Add to that, there were multiple versions of using the rating scales that were never clarified. The Primary Outcome Variable [HAM-D] was never reported.
In spite of milking the database, the worth certainly wasn’t in the reports on the primary outcome. The statement in red above leaves out a small detail, the patients disappeared at an impressive rate. I never could get the exact figures, but the graphic on the right is close. It was impossible to conclude how many actually remained at the end of each level. But the actual numbers didn’t come close to the numbers reported in the abstract. In addition, they changed rating scales in mid-stream. Add to that, there were multiple versions of using the rating scales that were never clarified. The Primary Outcome Variable [HAM-D] was never reported.
The data from NIMH-supported clinical trials are an important scientific resource. It is the view of NIMH that their full value can only be realized if they are made available, under appropriate terms and conditions, in a timely manner to the wider scientific community, provided that the confidentiality and privacy of study participants are protected. NIMH requires all investigators seeking access to data from NIMH-supported trials held by NIMH to execute and submit as their request the appropriate Data Use Certification pertaining to the trial. The datasets distributed by NIMH are referred to as "limited access datasets" because access is limited to qualified researchers who complete Data Use Certifications. The current listing of these datasets may be found on the Available Limited Access Datasets From NIMH Clinical Trials page.
Investigators wishing to request any of the above datasets must obtain and submit a Data Use Certificate (DUC) in order for NIMH to release the dataset. Please note that the DUC requires the institution’s Federal Wide Assurance (FWA) number and the signature of the institution’s official. To request a DUC, send an email to: nimhdatasets@mail.nih.gov. Be sure to specify the dataset that you are requesting.
To be honest i never really understood the STAR*D study. I mean i read it, and I read about the problems with it. I just couldn’t understand what the study was trying to say in the abstract.
According to the study
“CONCLUSIONS: When more treatment steps are required, lower acute remission rates [especially in the third and fourth treatment steps] and higher relapse rates during the follow-up phase are to be expected. Studies to identify the best multistep treatment sequences for individual patients and the development of more broadly effective treatments are needed.”
That seemed roughly consistent with what I would imagine. ‘Replase’ was drug dependence induced, a result of long term use possibly compounding whatever might have been wrong with the patient to begin with, or just by itself. That’s where we got the research term “Tardive dysphoria” from. The longer the drug use, the fewer the patients would ever recover.
“Tardive dysphoria: the role of long term antidepressant use in-inducing chronic depression.”
http://www.ncbi.nlm.nih.gov/pubmed/21459521
Only problem is that the abstract claimed an overall remission rate of 67%, and reccomended “Studies to identify the best multistep treatment sequences”. The 67% figure can’t be reproduced from the data that is available, and I thought this ‘multistep’ process clearly harmed more people then it helped!? There are more dropouts then remissions.. unless we’re ignoring the first set of dropouts and hiding that last number? I cou;ldn’t understand what the paper was trying to deduce and the numbers don’t match reality. 35 million dollars of tax money wasted so completely.
Usually authors who are reluctant to share data do it for a reason, their data doesn’t match their abstract. This is disturbing to come from a goverment department though, hiding science performed for public benefit paid for by tax dollars is just a very very bad sign.
If you can obtain a “Data Use Certificate”, things should be very interesting.
I know it’s off topic but you gotta help me Dr. Mickey, I need a shrink with a touch of gray about the temples…. The Veterans Admin has been defending itself in the past 24 hrs with statements that Mr Alexis was NOT being treated by the VA for psych problems. He came to the emergency room for INSOMNIA and we gave him SLEEPING PILLS. That’s all folks. (The system being so f–ed up that a guy showing up twice in a week to the ER for “insomnia” starts to look like normal behavior …)
This WashPost article is all over Twitter. Apparently the current crop of docs views Trazodone as a general purpose sleeping pill. Well of course, it’s so cheap! How could it possibly be a major psychiatric drug?
http://www.washingtonpost.com/national/health-science/trazodone-antidepressant-used-by-aaron-alexis-described-as-very-safe/2013/09/18/4336c044-20ae-11e3-966c-9c4293c47ebe_story.html?wprss=rss_health-science
Lord help us …
protecting “trade secrets?”
“Heart Gadgets Test Privacy-Law Limits” WSJ
“The U.S. has strict privacy laws guaranteeing people access to traditional health files. But implants and other new technologies—including smartphone apps and over-the-counter monitors—are testing the very definition of medical records.”
http://online.wsj.com/article/SB10001424052970203937004578078820874744076.html
That paper on “tardive dysphoria” does not take into account withdrawal syndrome misdiagnosed as relapse in studies where antidepressants were discontinued. Virtually no such studies contain protocols to distinguish withdrawal syndrome from relapse.
(Withdrawal syndrome is more common and severe the longer the patient is medicated.)
Same with STAR*D. It switched more than 4,000 people from drug to drug and contained no protocols to identify withdrawal syndrome. The STAR*D study reported zero cases of withdrawal syndrome. The only post-discontinuation instrument used were depression scales (of questionable validity, see
http://1boringoldman.com/index.php/2011/04/09/the-appearance-of-conflict-of-interest/
(Dr. Mickey wrote another post about a paper debunking the telephone survey used in STAR*D — does anyone have the link?)
When taken with a P450 cyp 2D6 inhibitor, trazodone’s active metabolite MCPP can cause agitation, panic, hallucinations — you name it, it’s a nasty molecule. Drug-drug interactions with trazodone are quite common.
Muchas Gracias Altostrata! You wouldn’t happen to have a link to any of that Trazodone interaction stuff would you?
Back in the 80’s they used to actually hospitalize people to start or adjust a drug like trazodone. Now it’s to be handed out like candy in the ER to anyone who says they can’t sleep?
QIDS-S-telephone found here:
http://1boringoldman.com/index.php/2013/05/31/a-lot-better-than-this/
Thanks, a-non. That one’s a keeper.
About trazodone and MCPP:
World J Biol Psychiatry. 2009;10(4 Pt 2):682-5. doi: 10.1080/15622970902836022.
http://www.ncbi.nlm.nih.gov/pubmed/19384678
Trazodone generates m-CPP: in 2008 risks from m-CPP might outweigh benefits of trazodone.
Kast RE.
Source
Department of Psychiatry, University of Vermont, Burlington, VT 05401, USA. rekast at email.com
Abstract
Since deleterious effects of m-CPP, the primary catabolic metabolite of trazodone, were last reviewed 2 years ago, research data continue to accrue showing that clinically significant levels of m-CPP (a) are generated in patients using trazodone for sleep and (b) are present 24 h a day and (c) have potentially serious ill effects. This commentary argues that the documented potential for harm and multiple risks of m-CPP outweigh potential benefits of trazodone, given the development and marketing of many safer alternatives since trazodone’s introduction in the 1980s.
Other papers:
http://www.ncbi.nlm.nih.gov/pubmed/9616194
http://www.ncbi.nlm.nih.gov/pubmed/9836023
http://www.ncbi.nlm.nih.gov/pubmed/15916857
http://www.ncbi.nlm.nih.gov/pubmed/7870844
http://www.ncbi.nlm.nih.gov/pubmed/2287489
Trazodone is metabolized by cyp 3A4 to MCPP. MCPP is metabolized by cyp 2D6. MCPP is used as an anxiolytic in experiments.
Despite this, MCPP is ingested as a recreational drug to induce hallucinations http://www.who.int/medicines/areas/quality_safety/5.3cmCPPpre-review.pdf? (pdf): “1-(3-chlorophenyl)piperazine (mCPP) is a piperazine derivative with stimulant (including
euphoric) and hallucinogenic properties. mCPP has never been licensed as a medicine but is a known metabolite of some anti-depressants (trazodone, nefazodone, enziprazole and
etoperidone) and a tranquillizer (mepiprazole)….”
Trazodone drug interactions http://www.drugs.com/drug-interactions/trazodone.html