With the Paxil Study 329 paper, our problem getting it in print didn’t have to do with journal shopping, it had to do with a tough love review process and a year of uncertainty that went with it. It was a top journal [British Medical Journal Impact Factor 17.445] and I’m glad it’s there. The recent Citalopram paper did have to do a lot of journal shopping hopping [see background notes], moving from the Journal of Affective Disorders [Impact Factor 3.383] to JAMA Psychiatry [Impact Factor 12.008] to the Acta Psychiatrica Scandinavica [Impact Factor 5.605] to the International Journal of Risk and Safety in Medicine [Impact Factor 0.86]. Quite a journey.
A few months back, I was writing about an elaborate KOL-rich campaign by Takeda and Lundbeck to get FDA Approval for Vortioxetine [Brintellix®] in Cognitive Dysfunction in Major Depressive Disorder [see indications… and more vortioxetine story…]. I thought it was a commercially driven attempt [indication creep] and the science was woefully lacking. I was pleased that the FDA later agreed [a parable…] and didn’t approve the indication. Prior to that, my only encounter with Vortioxetine [Brintellix®] was a industry-produced review article in the Journal of Clinical Psychiatry [Impact Factor 5.498]:
by Alan F. Schatzberg, Pierre Blier, Larry Culpepper, Rakesh Jain, George I. Papakostas, and Michael E. Thase.Journal of Clinical Psychiatry 2014 75[12]:1411–1418.
Six clinicians provide an overview of the serotonergic antidepressant vortioxetine, which was recently approved for the treatment of major depressive disorder in adults. They discuss the pharmacologic profile and receptor-mediated effects of vortioxetine in relation to potential outcomes. Additionally, they summarize the clinical trials, which demonstrate vortioxetine’s efficacy, and discuss findings related to safety and tolerability that have high relevance to patient compliance.
by Lisa Cosgrove, Steven Vannoy, Barbara Mintzes, and Allen Shaughnessy
by Chi-Un Pae, Sheng-Min Wang, Changsu Han, Soo-Jung Lee, Ashwin A. Patkar, Praksh S. Masand, and Alessandro SerrettiJournal of Psychiatry and Neuroscience. 2015 40[3]: 174–186.
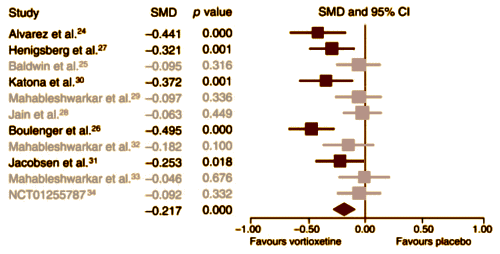
Background: Vortioxetine was approved by the U.S. Food and Drug Administration [FDA] in September 2013 for treating major depressive disorder [MDD]. Thus far, a number of randomized, double-blind, placebo-controlled clinical trials [RCTs] of vortioxetine have been conducted in patients with MDD. We performed a meta-analysis to increase the statistical power of these studies and enhance our current understanding of the role of vortioxetine in the treatment of MDD.Methods: We performed an extensive search of databases and the clinical trial registry. The mean change in total scores on the 24-item Hamilton Rating Scale for Depression [HAM-D] and the Montgomery–Åsberg Depression Rating Scale [MADRS] from the baseline were the primary outcome measures. The secondary efficacy measures were the response and remission rates, as defined by a 50% or greater reduction in HAM-D/MADRS total scores and as a score of 10 or less in the MADRS and 7 or less in the HAM-D total scores at the end of treatment.Results: We included 7 published and 5 unpublished short-term [6–12 wk] RCTs in our meta-analysis. Vortioxetine was significantly more effective than placebo, with an effect size [standardized mean difference [SMD]] of ?0.217 [95% confidence interval [CI] ?0.313 to ?0.122] and with odds ratios [ORs] for response and remission of 1.652 [95% CI 1.321 to 2.067] and 1.399 [95% CI 1.104 to 1.773], respectively. Those treated with vortioxetine did not differ significantly from those treated with selective norepinephrine reuptake inhibitors/agomelatine with regard to the SMD of the primary outcome measure [0.081, ?0.062 to 0.223] or for response [OR 0.815, 95% CI 0.585 to 1.135] and remission [OR 0.843, 95% CI 0.575 to 1.238] rates. Discontinuation owing to lack of efficacy [OR 0.541, 95% CI 0.308 to 0.950] was significantly less common among those treated with vortioxetine than among those who received placebo, whereas discontinuation owing to adverse events [AEs; OR 1.530, 95% CI 1.144 to 2.047] was significantly more common among those treated with vortioxetine than among those receiving placebo. There was no significant difference in discontinuation rates between vortioxetine and comparators owing to inefficacy [OR 0.983, 95% CI 0.585 to 1.650], whereas discontinuation owing to AEs was significantly less common in the vortioxetine than in the comparator group [OR 0.728, 95% CI 0.554 to 0.957].Limitations: Studies examining the role of vortioxetine in the treatment of MDD are limited.Conclusion: Although our results suggest that vortioxetine may be an effective treatment option for MDD, they should be interpreted and translated into clinical practice with caution, as the meta-analysis was based on a limited number of heterogeneous RCTs.
Effect Size of the Primary Outcome Variable
[adapted for blog]
This is fascinating, 1bom had you ever seen this?
http://post.queensu.ca/~sismondo/ghosts.pdf
The discussion section is amazing.
1bym,
So, the Ghosts in the Machine is the most depressing piece yet…