Posted on Friday 2 September 2016
Contributors and sources: The author worked from 1994 to 2012 as an independent consultant in the pharmaceutical, marketing and medical communications sectors, with extensive experience of market analysis, strategic communications planning, publications planning and medical writing. He has also studied the interface between academia and commerce from the standpoints of anthropology and publications policy.Competing interests: I have read and understood BMJ policy on declaration of interests and declare the following interests: Between 1994 and 2012 most of my income came from consultancy and writing services provided to pharmaceutical corporations, either directly or via marketing agencies. In 2015 I acted as a paid expert witness on behalf of the plaintiffs in a US federal legal action against a drug company. I have valued friendships and acquaintances in the corporate pharmaceutical and marketing sectors. I consider myself to be a supporter of innovative pharmaceutical research, but a critic of some forms of marketing. I was solely responsible for all aspects of conception, design, and writing of this article and am the guarantor.
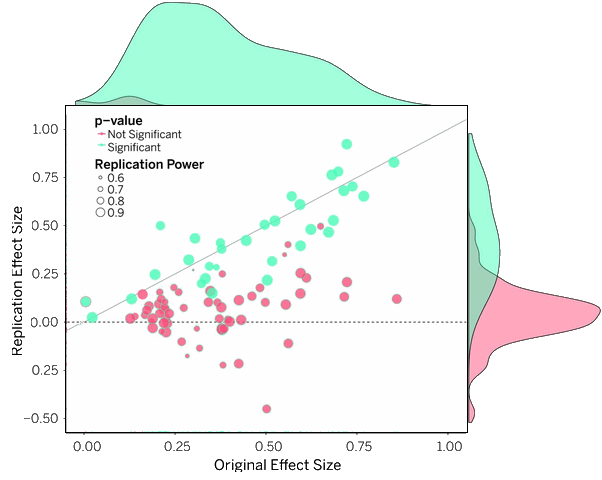
by Alastair MathesonBritish Medical Journal 2016 354:i4578.During the past decade, the pharmaceutical publications trade has campaigned to persuade medicine, journals, ethicists, and the media that it is opposed to ghostwriting. Yet industry practices have changed little, and commercial drafting of clinical trial reports, consensus statements, and reviews that are authored by recruited academics remains routine. Here, I show that industry’s opposition is based on a redefinition of the term ghostwriting that obscures the continued, widespread use of the practice as originally defined in medical journal literature…
Outside medicine, a ghostwriter is “a person whose job it is to write material for someone else who is the named author.” Drug companies and their agencies use writers to develop articles for academic authors, and in the 1990s and early 2000s this was commonly understood as ghostwriting, both by journal editors and the pharmaceutical publications trade. But today, the trade promotes an alternative definition, whereby writers are not classified as ghosts if they are named in a footnote…
The Global Alliance of Publication Professionals, a trade advocacy unit, states: “A ghostwriter is someone who writes a paper, but whose name does not appear on the paper.” Simply by acknowledging writers for editorial or writing “assistance”—a practice always widespread—commercially written literature is by these definitions freed from the egregious ghostwriting label. Consider, for instance, two commercially drafted studies of paroxetine, both criticised for using ghostwriting to produce allegedly biased content. One, study 352, mentions no writer but the other, the notorious study 329, discloses at the end of a tract of small print that “Editorial assistance was provided by Sally K Laden, MS.” By the traditional definition, both articles are ghostwritten, but according to the trade definitions quoted above, Study 329 is not, even though the medical writer’s credit is inconspicuous…
What Matheson calls rebranding goes much further. For example, in Paxil Study 329 and Celexa CIT-MD-18, they redefined the meaning of a priori to before the blind is broken rather than before the study begins. Redefining Outcome variables is another common form of rebranding. But that’s just one of the tools of the trade in the process of misreporting the results of these clinical trial reports [I wonder if there’s a handbook somewhere that catalogs all the various tools and techniques available to misreport clinical trial reports?].
Matheson in an insider who knows the tools of the trade, and as such, he deserves to be listened to carefully – particularly when he suggests what should be done about all of this. He proposes a number of thoughtful standards for accurate attribution of Authorship, focusing his attention on the International Committee of Medical Journal Editors [ICMJE]. But I’m not sure that would change things any more than the many other attempts at reform – COI declarations, acknowledging funding sources, naming the ghostwriters, the Sunshine Act, etc. They’re all proxies. They don’t get at the central issue directly, which is accurate information about the trial results.



![[Andrew Medichini/Associated Press] [Andrew Medichini/Associated Press]](http://1boringoldman.com/images/rio2016.jpg)
 Living in England in 1972, we got to spend two weeks at the Munich Olympic games. And in spite of the craziness of the terrorist attack, those days remain a peak experience in my memory theater. Then in 1996, the games came to our house [Atlanta]. But even in all those years where they only played out on a television screen, it has always been the same – something special. rio·2016 is no exception. The Olympics games are just one of the best ideas humankind ever came up with…
Living in England in 1972, we got to spend two weeks at the Munich Olympic games. And in spite of the craziness of the terrorist attack, those days remain a peak experience in my memory theater. Then in 1996, the games came to our house [Atlanta]. But even in all those years where they only played out on a television screen, it has always been the same – something special. rio·2016 is no exception. The Olympics games are just one of the best ideas humankind ever came up with…
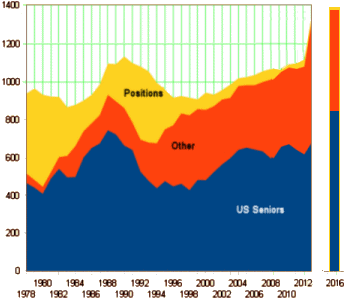 Back then, the need was for physicians [psychiatrists] to staff the mental health centers that were an essential ingredient of the Community Mental Health enterprise. It was actually a good plan in my estimation, though by the time I arrived in the mid·1970s, it was in its waning days – collapsing under the weight of under·funding, under·staffing, and stripped of the necessary hospital backup for stabilization. With the coming of the DSM-III, brain science, and the medicalization of psychiatry, there was the kind of heyday new paradigms often bring. But in general, the relative number of US graduating seniors choosing psychiatry is basically flat compared to other medical specialties.
Back then, the need was for physicians [psychiatrists] to staff the mental health centers that were an essential ingredient of the Community Mental Health enterprise. It was actually a good plan in my estimation, though by the time I arrived in the mid·1970s, it was in its waning days – collapsing under the weight of under·funding, under·staffing, and stripped of the necessary hospital backup for stabilization. With the coming of the DSM-III, brain science, and the medicalization of psychiatry, there was the kind of heyday new paradigms often bring. But in general, the relative number of US graduating seniors choosing psychiatry is basically flat compared to other medical specialties.
 I’ll have to admit that the notion of seeing a suicidal patient and offering them a sniff of some version of Special K as a treatment strikes me as borderline ludicrous, hard for me to say with a straight face – but stranger things have happened. That’s not why I’m writing about it. Remember, this is Johnson and Johnson, the people who gave us TMAP and all of the Risperidal® sheenanigans. The CEO is still Alex Gorsky – West Point graduate and lifelong Boy Scout, who brought in Billions with all kinds of hanky-panky, flooding the literature with articles churned out by Excerpta Medica. Johnson and Johnson gladly paid the cost-of-doing-business fines of around $2.2 B – trivial in the face of their ill-gotten gains. And with Risperidal®, they were also first-on-the-market:
I’ll have to admit that the notion of seeing a suicidal patient and offering them a sniff of some version of Special K as a treatment strikes me as borderline ludicrous, hard for me to say with a straight face – but stranger things have happened. That’s not why I’m writing about it. Remember, this is Johnson and Johnson, the people who gave us TMAP and all of the Risperidal® sheenanigans. The CEO is still Alex Gorsky – West Point graduate and lifelong Boy Scout, who brought in Billions with all kinds of hanky-panky, flooding the literature with articles churned out by Excerpta Medica. Johnson and Johnson gladly paid the cost-of-doing-business fines of around $2.2 B – trivial in the face of their ill-gotten gains. And with Risperidal®, they were also first-on-the-market: 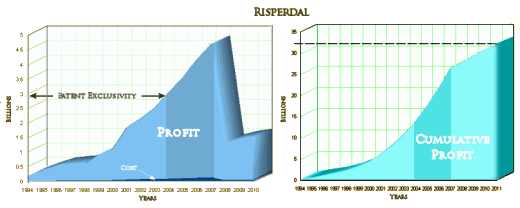
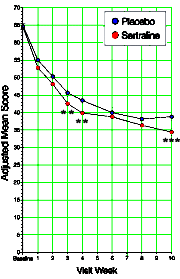 Figure 2. Weekly and Overall Adjusted Mean CDRS-R Scores
Figure 2. Weekly and Overall Adjusted Mean CDRS-R Scores This NEJM article sounds like Nero playing his violin while Rome burns to me. I can think of nothing in Medicine that comes even close the level of corruption achieved in the distortion of Clinical Trial results in the fifty years since the
This NEJM article sounds like Nero playing his violin while Rome burns to me. I can think of nothing in Medicine that comes even close the level of corruption achieved in the distortion of Clinical Trial results in the fifty years since the