This post is too long, too heavily referenced, and too detailed. I apologize for that up front. The question it raises seems simple and important to me, but you have to swim through a mighty murky swamp to find that question, and I don’t see any other way to get to it that isn’t too long, too heavily referenced, and too detailed. So proceed at your own risk...
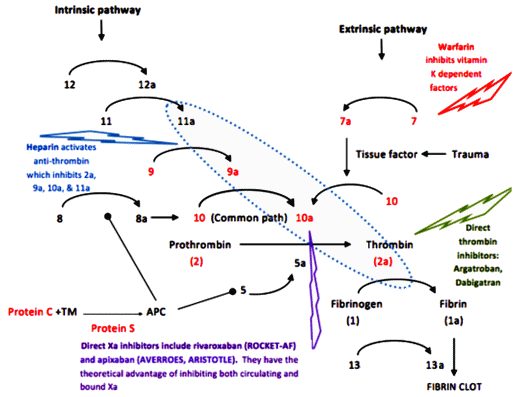
If you went to medical school, you probably shudder a bit seeing this figure – remembering staying up late at night drawing it over and over so it could be reproduced on some test the next day. It’s the cascade of things that have to happen to make the blood clot, and those numbers are the various clotting factors involved in the process. It’s right up there along with those similar diagrams of the Krebs Cycle that were so hard for the mind to hold onto. Over time, one remembers that there is such a cascade, but couldn’t reproduce it on demand. But what you do remember is how to turn the clotting machinery down for therapeutic reasons. During most of my medical life, there were two ways to anticoagulate a patient:
-
Heparin – a naturally occurring compound found in certain cells. As a drug, it has to be given by injection, acts immediately, is short acting [hours], and blocks the action of any number of clotting factors. It can be reversed [with Protamine Sulfate].
-
Warfarin [Coumadin] – Originally introduced as a Rat Poison, Warfarin interferes with the the metabolism [re-use] of Vitamin K which is necessary for the creation and action of a number of those factors [in red], specifically their binding of Calcium. It takes several days to work [produce a Vitamin K deficient state], is sensitive to dietary factors and many drugs, and has to be monitored with frequent [monthly] blood tests. It can be fairly quickly reversed with Vitamin K injections.
There are all kinds of medical situations where these drugs are used. One big one is Atrial Fibrillation, a disturbance of heart rhythm common in the elderly. Clots tend to form in the heart in this condition leading to stroke, so these patients routinely take anticoagulants as prophylaxis. They have to abide by food and drug restrictions, and get monthly blood tests while taking it. Oh yeah, avoid having a big car wreck! Inconvenient treatment, but it sure beats having a stroke.
A few years back, I ran across something new. A friend [with limited means] developed Atrial Fibrillation and was prescribed a drug I’d never heard of before. He was fretting over the cost, and I looked into it. It was one of the new ones, Pradaxa®, and it would’ve been a big out-of-pocket expense for a retired "man of the cloth." He was told that there weren’t the restrictions and you didn’t have to get all of those blood tests. It was "better." So I hit the Internet and learned about Xarelto® and Pradaxa®. The restrictions, the hassle of the blood tests, the dosage adjustments weren’t necessary sure enough, but underscore avoid having a big car wreck! two or three times, because at the time I was looking, there was no easy antidote except time. In the figure, their action is more specific, way down at the bottom of the cascade – and there was no off switch then
We had endlessly teased my friend and his wife, calling them Gypsies, because they were always on the road visiting many friends. But his doctor got him a discount somehow, so he opted for the new drug and had no problems, later dying from something unrelated. But in my searching around, I decided that these were "drugs of convenience" rather than "better." I have to admit that my negativity probably has to do with having spent some long nights when I was younger with several wreck victims on Coumadin who I doubt would’ve survived without that off switch.
Why is this here? While most of my blogs are about the clinical trials of psychiatric drugs, my major interest is in the distortion of trial data in medicine at large no matter where it happens. This example originally caught my eye because its Principle Investigator, Dr. Robert Califf, is our new head of the FDA. But this report adds another character of interest to the mix, Dr. Jeffrey Drazen, editor of the New England Journal of Medicine. Dr. Drazen’s recurrent pro-PHARMA leanings lately have earned him a place on my radar screen. So I thought I’d review my clotting before wading into this recent wrinkle in the story. Here’s the main article…
-
New York Times
by Katie Thomas
March 1, 2016
… which requires some background so it’s summarized again below. I’ve sprinkled some of the references along the way [note that an antidote is now in the mix!]. But first, what’s all the recent noise about? Set aside my concern about an antidote for a moment.
This is about something else. The FDA initially wasn’t going to approve it, but that’s not the issue here either.
-
by Manesh R. Patel, Kenneth W. Mahaffey, Jyotsna Garg, Guohua Pan, Daniel E. Singer, Werner Hacke, Günter Breithardt, Jonathan L. Halperin, Graeme J. Hankey, Jonathan P. Piccini, Richard C. Becker, Christopher C. Nessel, John F. Paolini, Scott D. Berkowitz Keith A.A. Fox, Robert M. Califf, M.D., and the ROCKET AF Steering Committee, for the ROCKET AF Investigators
New England Journal of Medicine 2011 365:883-891.
-
-
Now there’s a suit alleging damages and deaths, but that’s also not directly under the microscope here except as a force calling attention to the drug. There was a technological glitch in that a machine used to measure the clotting in patients on Coumadin during the study. It was recalled a couple of years after Xarelto® was approved.
It had under-estimated the clotting and since it had been used to adjust the doses for Coumadin, the concern was that the Coumadin subjects in the study might have been overmedicated, giving Xarelto® an unfair advantage in the trial. There’s no allegation that either the company [J&J and Bayer] or the Duke researchers had anything to do with that that I know of. But once it was discovered, it called the results of the study into question. So the the researchers reanalyzed the original data «warning! idiom alert» every whichaway from Sunday and concluded that the machine’s misbehavior didn’t change things. They published the reanalysis in the New England Journal of Medicine [editor Jeffrey Drazen].
-
BMJ 2015 351:h6431
-
by Manesh R. Patel, Anne S. Hellkamp, Keith A. A. Fox, for the ROCKET AF Executive Committee and Investigators
New England Journal of Medicine. 2016 374:785-788.
-
New York Times
by Katie Thomas
February 22, 2016
And my references are just for starters. Google News has enough articles to get you to the middle of the summer about what happens next. Now we’re back to the first article and to the part that matters.
New York Times
By KATIE THOMAS
MARCH 1, 2016
It is a startling accusation, buried in a footnote in a legal briefing filed recently in federal court: Did two major pharmaceutical companies, in an effort to protect their blockbuster drug, mislead editors at one of the world’s most prestigious medical journals? Lawyers for patients suing Johnson & Johnson and Bayer over the safety of the anticlotting drug Xarelto say the answer is yes, claiming that a letter published in The New England Journal of Medicine and written primarily by researchers at Duke University left out critical laboratory data. They claim the companies were complicit by staying silent, helping deceive the editors while the companies were in the midst of providing the very same data to regulators in the United States and Europe…
Duke and Johnson & Johnson contend that they worked independently of each other. Bayer declined to comment. And top editors at The New England Journal of Medicine said they did not know that separate laboratory data existed until a reporter contacted them last week, but they dismissed its relevance and said they stood by the article’s analysis. But the claim — that industry influence led to the concealing of data — carries echoes, some experts said, of an earlier era of drug marketing, when crucial clinical data went missing from journal articles, leading to high-profile corrections and a wave of ethics policies to limit the influence of drug companies on medical literature…
Last month the Duke researchers published an analysis in The New England Journal of Medicine and concluded that the problems with the device did not change the trial’s results. But some in the medical community questioned their findings because their method required them to essentially guess which groups of patients were more likely to be affected by the malfunctioning device. A better way to evaluate the device, other researchers said, would be to compare the device readings with test results that were done at a central laboratory. Investigators did that at two points in the trial, drawing blood from more than 5,000 of the patients who took warfarin and sending the samples for testing. The blood was taken 12 and 24 weeks after patients enrolled in the trial. But the Duke researchers made no mention of the lab data in their letter. In an interview, journal editors said they did not know about the lab data until last Tuesday, when a reporter for The New York Times asked them about it. “At the time we published the letter, we didn’t know that it existed,” said Dr. Jeffrey M. Drazen, editor in chief of The New England Journal of Medicine…
In a footnote, the lawyers said that during the process of vetting the Duke researchers’ letter, a peer reviewer asked about the existence of lab data that would allow a comparison with the device’s readings. “Despite being provided this opportunity to respond to the peer reviewers,” the lawyers said, the “defendants remained silent on this point, thereby misleading the NEJM.” Dr. Drazen confirmed that a peer reviewer, whose identities are kept confidential, had asked about such data, but said the editors had rephrased the question to ask whether such data was available throughout the course of the trial. Duke then answered no, he said. The letter’s three authors, two from Duke and one affiliated with the University of Edinburgh in Scotland, declined to comment, as did a spokesman for Duke…
So far, the New England Journal of Medicine and the European Medicines Agency [who reanalyzed this extra data] have concluded that the original results stand in spite of the machine glitch, however:
Dr. Steven Nissen, a cardiologist at the Cleveland Clinic, served on the Food and Drug Administration advisory panel that voted to approve Xarelto in 2011. He was one of two members who voted against the drug. He expressed doubt that any after-the-fact analysis would give doctors and patients answers. “Given the fact that the device was inaccurate, there is no way anybody can tell you what would have happened in the trial,” he said.
Dr. Nissen is the guy that busted Vioxx [and I tend to think he’s right, or at least not wrong, in this case]. So here’s that simple question. Once the question was raised about the machine, the obvious fallback position would’ve been to look at that independent subset of the data analyzed in a different way [not that machine] at 12 and 24 months. Duke didn’t do that. Was that because they didn’t know about those samples? If they didn’t know, why not? It was their study. J&J and Bayer did know, and gave that data to the EMA and FDA. Should they have alerted Duke about it? Is this a story about something falling through the cracks? Or did somebody somewhere see what was happening and let it fall through anyway? Is Dr. Nissen right, that the truth is that the whole thing was invalidated the day they discovered the glitch in the machine? Is this an example of "Shit happens" or of somebody misbehaving? Even if the EMA and the NEJM are right that it didn’t change the study’s results, is all the bruhaha invalidated or do the questions of mafeasance remain? And for that matter, should that extra data have alerted Duke and the sponsors along the way that the machine was misfiring by comparison.
While I can’t answer these questions, I want to make two points. First, if we had real Data Transparency we wouldn’t be asking many of those questions, and we’d likely know the answers to the ones that remain by now. Second, Xarelto® brought in $2B last year in profits. That’s a big part of the whole story from beginning to end [about this drug of convenience] and how all these questions are playing out. If that were not in the mix, I expect traditional-medical-do-no-harm-wisdom would’ve should’ve put this drug on hold as soon as the machine problem showed up until things became clear. At least that’s what I think. Oh yeah, and what about that rephrasing in red above?…


 I belatedly became aware of the magnitude of the Conflict of Interest [COI] problems in our literature in 2008 with the revelations of Senator Chuck Grassley and his investigator Paul Thacker. But in the years since, I’ve realized that by then what was once unthinkable had already verged on routine, particularly in the industry-sponsored clinical drug trials, particularly in psychiatry. Recently, we’ve seen several articles advocating an even further laxing of COI standards. In this editorial, Dr. Fava goes beyond just decrying the COI problem – he lays out how it came into being and the forces that sustain it. Then he talks about what we can do about the problem…
I belatedly became aware of the magnitude of the Conflict of Interest [COI] problems in our literature in 2008 with the revelations of Senator Chuck Grassley and his investigator Paul Thacker. But in the years since, I’ve realized that by then what was once unthinkable had already verged on routine, particularly in the industry-sponsored clinical drug trials, particularly in psychiatry. Recently, we’ve seen several articles advocating an even further laxing of COI standards. In this editorial, Dr. Fava goes beyond just decrying the COI problem – he lays out how it came into being and the forces that sustain it. Then he talks about what we can do about the problem…