Posted on Tuesday 29 September 2015
What Happened to those Suicidal in Study 329?In May 2014, the RIAT team asked GSK what the children who became suicidal in the course of Study 329 have since been told. The consent form says that anyone entering the study would be treated just the way they would be in normal clinical practice.
In Study 329, the children taking imipramine were by design force titrated upwards to doses of the order of 300 mg, which is close to double the dose of imipramine given in adult trials by GSK or in normal clinical practice. In normal clinical practice it would be usual to inform somebody who had become suicidal on an SSRI that the treatment had caused their problem.
As I have mentioned in my earlier letters, it is standard in clinical trials carried out according to good clinical practice guidelines for our trial investigators and treating physicians to be responsible for patients’ medical care during and after a trial. This would include the management of any adverse experiences that arise during the trial. Being closest to patients’ medical histories, they are best placed to do this and we are confident of their commitment to provide the care patients need.This may be the first recorded appeal to the use of the Learned Intermediary doctrine in clinical trial settings. One lesson of the 329 story is that without access to data, the learned intermediary doctrine is supremely dangerous for patients – and for doctors…
Sitting in the midst of a Maelstrom in 2004 when New York State were suing GSK for Fraud, faced with demands to “modify” Study 329, on June 13 Marty Keller emailed some of his co-authors to get a united position about what they would say to GSK about the modification:
… that [it must be] 100% clear in this paper that there is no way to read it and think that 329 is being criticized and that it was not written with complete integrity and accuracy given the data we had and should have had….. We also want it to be crystal clear that any new data or analyses, case report forms, narratives etc. you have worked with since 329 was published, was not made available to the 329 investigator’s by SK, otherwise we could look foolish, naïve, incompetent or “biased” (the most likely accusation that will be made) to present things in a way that was favorable to SK, disregarding our responsibility to the proper scientific method, to the public, children and their families.So as MK & GSK see it, while the Learned Intermediary Doctrine rules, he and his colleagues have a responsibility to their patients. Someone needs to do the right thing by the Paxil 12…
International Business TimesBy Amy NordrumSeptember 29 2015
Except for the recent Swedish study [see an innovative design… ], the population studies haven’t detected the suicidality Adverse Effects. I think this article probably speaks to why. Most patients who have this kind of reaction either recognize it or are told about it by others and stop the medication and move on. Thus, there’s no way for them to show up in a population survey. I was surprised at the actual incidence in Paxil Study 329 – greater than 10%. This is one place where the clinical trial, carefully reported, can tell us something missed in other venues [because the subjects are so closely watched].
GSK voluntarily published the Paxil trials through a new program called RIAT, which stands for Restoring Invisible and Abandoned Trials. RIAT aims to nudge clinical research and drug development toward greater transparency and data sharing, and GSK was the first company to participate.



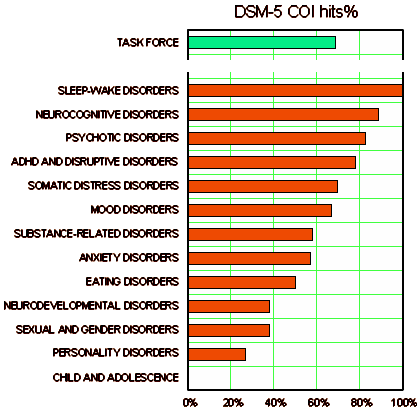 Similar experts with [financial] PHARMA COI are everywhere: CME presentations; Speaker’s Bureaus; Review Articles; the list gets longer by the year. A recent NEJM series [
Similar experts with [financial] PHARMA COI are everywhere: CME presentations; Speaker’s Bureaus; Review Articles; the list gets longer by the year. A recent NEJM series [

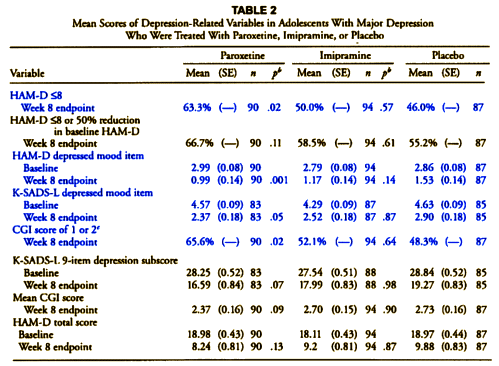
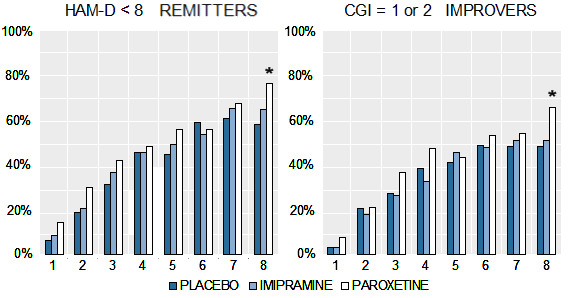
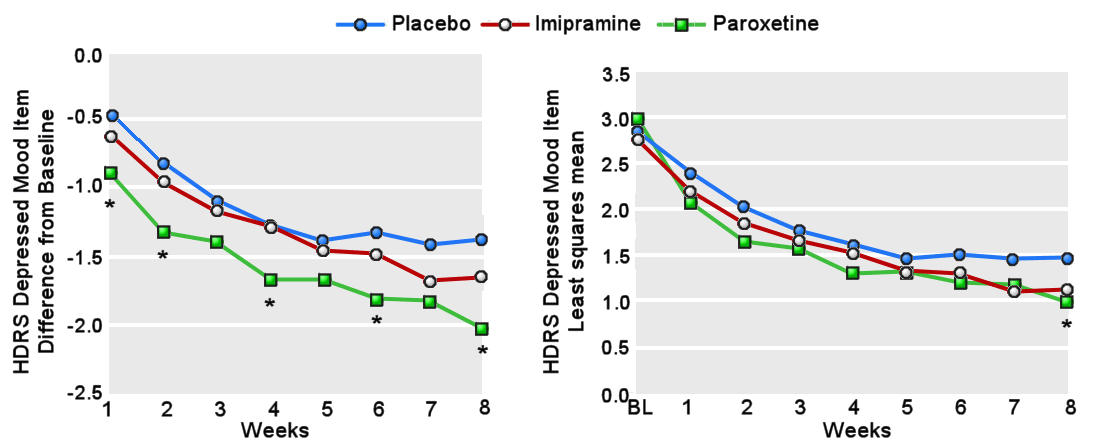
 In the case of a study designed to advance the treatment of depression in adolescents, it seems strange to have picked imipramine 200-300mg per day as a comparator, unusual to have left the continuation phase unpublished, odd to have neglected to analyse the taper phase, dangerous to have downplayed the data on suicide risks and the profile of psychiatric adverse events more generally and unfortunate to have failed to update the record in response to attempts to offer a more representative version of the study to those who write guidelines or otherwise shape treatment.
In the case of a study designed to advance the treatment of depression in adolescents, it seems strange to have picked imipramine 200-300mg per day as a comparator, unusual to have left the continuation phase unpublished, odd to have neglected to analyse the taper phase, dangerous to have downplayed the data on suicide risks and the profile of psychiatric adverse events more generally and unfortunate to have failed to update the record in response to attempts to offer a more representative version of the study to those who write guidelines or otherwise shape treatment.