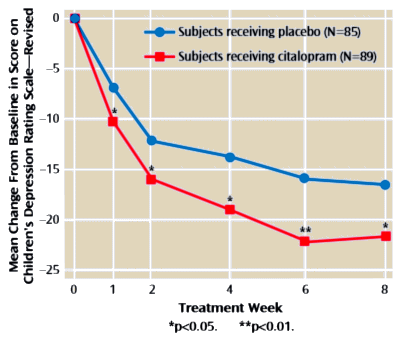
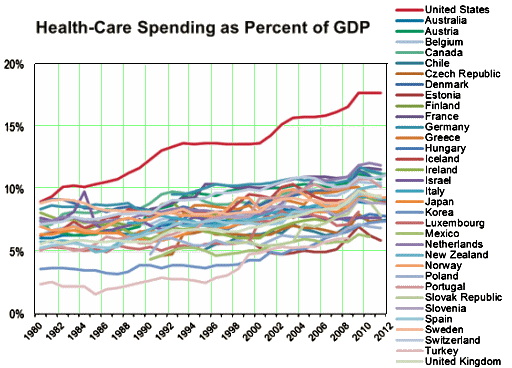
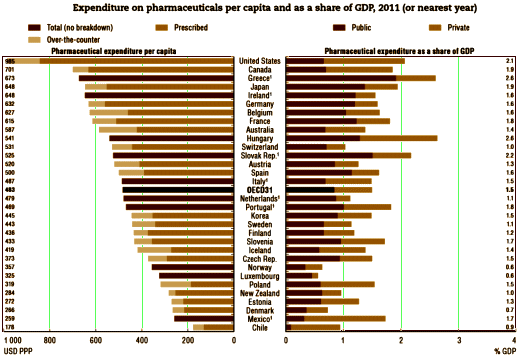
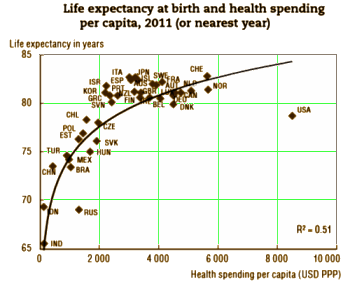
“APA has a role in shaping what future psychiatric practice looks like,” Schatzberg stated. “More needs to be done now if we are to have new treatments in the next decade for patients with psychiatric disorders.”
PsychiatricNews
October 17, 2014
As development of drugs to treat psychiatric disorders lags behind that of drugs for other illnesses, a recent study published in Psychiatric Services in Advance sheds light on why the pipeline for psychotropic medicines is nearly empty.
Researchers from Brandeis University and Truven Health Analytics led an investigation of the current state of psychotropic drugs in the pipeline and potential barriers that may keep these drugs from reaching distribution in the United States… The analysis showed that the pipeline for psychotropic drug development — 99 clinical trials were included — is limited, with little product innovation evident. Most of the examined drugs were a combination of existing of U.S. Food and Drug Administration-approved medicines or individually approved medicines that were being tested for new indications or delivery-system approaches [such as an injectable version that is similar to an approved oral form]. Only three drugs differed substantially from existing drugs…
In an interview with Psychiatric News, Alan Schatzberg, M.D., a professor of psychiatry at Stanford University and former APA president, said that the departure by pharmaceutical companies to develop innovative psychotropic medicines could result in serious problems for the field of psychiatry, especially for patients. “There is a number of initiatives by various organizations to help with this problem, including the European College of Neuropsychopharmacology, which is working with companies to provide investigators with compounds that have been shelved, and NIMH’s Research Domain Criteria [RDoC], which promotes research on specific [and new] biological targets," he said. Schatzberg emphasized that it will take a concerted effort on the parts of governmental agencies, industry, as well as APA to advocate for investment and innovative psychiatric drug development. “Silence will not be helpful to our patients,” he concluded…
Since it became apparent in the summer of 2011 that the pharmaceutical industry was abandoning CNS drug development, there has been a frantic level of activity in the halls of psychiatry. For the previous two decades, organized and academic psychiatry had occupied itself with brain research and testing, commenting on, [and promoting] the CNS drugs that flowed from the industrial pipeline. After a period of panic and attempts to re-engage PHARMA, two threads emerged: changing the role of psychiatrists [
the sequel I…] coming from the APA leadership; and relocating CNS drug development and research to various public institutions, shepherded by NIMH Director, Tom Insel. Over the last several years, in a series of blog posts Dr. Insel has described a number of strategies including the NIMH RDoC [
Research Domain Criteria] and many others now under a Translational Science umbrella in the NIH. If you check out the links, most will be familiar to anyone who periodically checks in with Dr. Insel’s blog:
Translational Science started its life as a concept meant to focus research on current medical needs and speed the research findings from the "bench to the bedside." The problem that there were scant findings to translate didn’t seem to matter, and the term appears to have morphed into meaning "anything that people think is a good idea" – a politically correct tag like "evidence-based medicine." Among the NCATS strategies, one stands out as a new addition – the IDG Project, AKA, Illuminating the Druggable Genome [an tongue-twister for the ages]:
Med Check
PsychiatricNews
by Vabren Watts
October 17, 2014
According to the National Institutes of Health [NIH], as many as 3,000 genes express proteins whose molecular actions could be altered by medicines, yet only 10 percent of these “druggable genes” are targeted by drugs that have been approved by the Food and Drug Administration [FDA].
The NIH recently announced the launch of Illuminating the Druggable Genome [IDG], a three-year pilot project to explore poorly understood genes that have the potential to be modified by medicines. The IDG will target understudied genes of four important protein families that may be affected by medications — nuclear receptors, ion channels, protein kinases, and G-protein coupled receptors.
“We have a gap in the drug-development pipeline between what gene activities we know could be modified by medication and what currently is targeted,” said James Anderson, M.D., Ph.D., director of the NIH Division of Program Coordination, Planning, and Strategic Initiatives. “By focusing on understudied genes, we hope to find potential targets for medications to treat or cure some of our most burdensome diseases — and then share what we learn so that all can build on this knowledge.”
Primary funding for pilot awards is coming from the NIH Common Fund, which supports high-impact pioneering research in all divisions of NIH. Institutions granted awards will thoroughly investigate potential gene targets and share what they learn on a public resource that will help the larger scientific community build on the findings through basic research and clinical translation.
… and from the NCATS:
Results from the Human Genome Project revealed that the human genome contains 20,000 to 25,000 genes. A gene contains [encodes] the information that each cell uses to make [express] a protein, which is essential for the body to function properly. Abnormal protein expression is associated with many human diseases, which makes proteins key targets for therapeutic agents…
Approximately 3,000 genes are considered part of the “druggable genome”, a set of genes encoding proteins that scientists can or predict they can modulate using experimental small molecule compounds. Yet, only about 10 percent of these genes encode proteins that have been targeted successfully by an approved drug. Therefore, a large number of proteins remain for scientists to explore as potential therapeutic targets. The vast majority of the druggable genome encodes four key protein families: G-protein-coupled receptors, nuclear receptors, ion channels and kinases…
By expanding the potential therapeutic space through the IDG program, NIH is clearing a path for more efficient disease-related research and more effective treatments for patients.
There’s an unexamined inertia of motion in this narrative. The system apparently requires an endless influx of new drugs, and widespread panic ensued when that flow was interrupted, evoking these radical efforts to restore it. In fact, the majority of that stream of CNS medications for the two decades after 1987 [Prozac] wasn’t really new – but rather a set of variations on themes from the 1950s windfall of psychotropic drugs, engineered to be better tolerated. We didn’t hear much about the old-ness of these drugs, at least not in the foreground, until the supply was exhausted. Then we heard of little else, and the gears started whirring overtime for new, novel, innovative strategies to find new targets for CNS drug development.
I did notice along the way that the last new better drug became the next old obsolete drug with some regularity. And with that came the illusion that the drugs were improving [I would now see it as more determined by patent life and advertising]. And in those salad days, psychiatry had developed a sizable commentator class – a group of academics who commented on the drugs, talked about what was coming next, wrote about the neurobiology of this or the psychobiology of that regularly. In my mind, I called it future-think, with the good stuff always lying just around the corner, but it never occurred to me that a house of cards might tumble if the march of the new ever came to an end. So now we’re illuminating the druggable genome, repurposing existing drugs, building neuro-chips for in vitro assays, and revising diagnoses to fit drug effects [RDoC] in an attempt to revitalize the previous flow of new treatments.
There was another quote in that article about the 2012 Pipeline Summit that has also stayed with me:
“There are huge unmet clinical needs in mental disorders and addiction. There should be tremendous interest in this area, but there is not.”
I thought that was an incredibly naive comment. One could say that about a million things in medicine. The comment implies that "if you need it, it will come". Most scientific discovery doesn’t work that way. It’s closer to, "don’t push the river, it runs by itself." There are places where mounting a huge effort in science speeds up the process, but those are areas where there’s a clear direction, and the task is working out the practical details [Manhattan Project, NASA, Salk Vaccine, DARPA, etc]. Their quest for genuinely new psychopharmacology starts from near zero. And the pharmaceutical industry has a tremendous interest; has been at it for years; and just couldn’t find a thread to pull that lead them anywhere. So whether you agree that new symptomatic CNS drugs are a critical national priority or not, the likelihood of locating them [if they indeed exist] may not be enhanced by NCATS, the RDoC, or any other directed research under NIH/NIMH martial law. In fact, this forced march might squeeze out the guy who would notice something odd on a dirty petri dish [Alexander Fleming Discovers Penicillin].
There’s little to assure us that these efforts are not motivated by some desperate attempt to revitalize and perpetuate the KOL/PHARMA Camelot that has long past its prime – another grant-driven effort that will attract the same tired researchers who have haunted the grant-recipient rolls for several decades with little to show for the dollars spent. Frances Collins [Dr. Genome] and Tom Insel [Dr. Clinical Neuroscience] have their fingerprints all over these programs. Isn’t it about time for them to pass the reins to some new blood whose leadership styles are less controlling and who have a better eye for picking independent and creative scientists to follow and support [rather than direct and micromanage]?







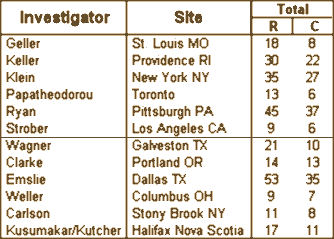 In the case of Dr. Carlson, Stony Brook was in the group of sites added to Paxil Study 329 late because of slow recruitment and accounted for only 11 subjects in the trial. She’s not mentioned in any of the subpoenaed documents that I know of. Apparently it was simply a source of revenue for Stony Book, and another paper to add to her CV. But that’s the whole point. Other than the two SKB/GSK employees who directed the show creating the paper with ghost-writer Sally Laden, the actual non-involvement was true for the twenty academic authors whose names were on that author‘s list. They lent their academic credentials and the reputations of their institutions to the paper, a ticket insuring that it would be accepted in a prestigious journal and read by colleagues who respected those honorifics, without really participating in its creation. But even worse, in the decade during which the paper has been universally vilified, not one of those authors has come forward and said a word. They continue to occupy high places, adding an obvious irony to Carlson’s comment, "Get here however you can!"
In the case of Dr. Carlson, Stony Brook was in the group of sites added to Paxil Study 329 late because of slow recruitment and accounted for only 11 subjects in the trial. She’s not mentioned in any of the subpoenaed documents that I know of. Apparently it was simply a source of revenue for Stony Book, and another paper to add to her CV. But that’s the whole point. Other than the two SKB/GSK employees who directed the show creating the paper with ghost-writer Sally Laden, the actual non-involvement was true for the twenty academic authors whose names were on that author‘s list. They lent their academic credentials and the reputations of their institutions to the paper, a ticket insuring that it would be accepted in a prestigious journal and read by colleagues who respected those honorifics, without really participating in its creation. But even worse, in the decade during which the paper has been universally vilified, not one of those authors has come forward and said a word. They continue to occupy high places, adding an obvious irony to Carlson’s comment, "Get here however you can!" Besides being an index of the strength of an effect, it also normalizes things so different studies can be compared. The usual range is 0.25 = weak, 0.50 = moderate, and 0.75 = strong. So what’s with effect size=2.9? It doesn’t make any sense. So I went back and pulled the whole paper to calculate it myself. Curious George, I guess…
Besides being an index of the strength of an effect, it also normalizes things so different studies can be compared. The usual range is 0.25 = weak, 0.50 = moderate, and 0.75 = strong. So what’s with effect size=2.9? It doesn’t make any sense. So I went back and pulled the whole paper to calculate it myself. Curious George, I guess…